Abstract
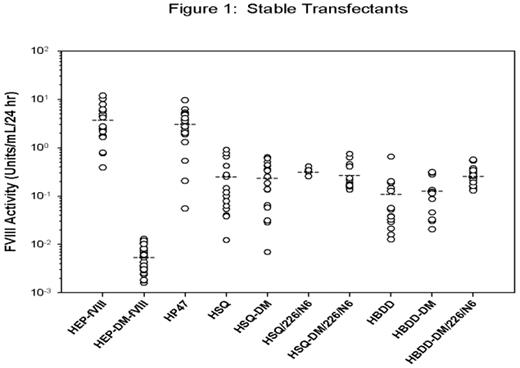
A major obstacle for gene therapy of hemophilia A is the achievement of adequate factor VIII (fVIII) expression. Bioengineering strategies have targeted specific sequences within human fVIII that are thought to be responsible for its generally poor expression. Specific amino acid substitutions, L303E/F309S herein referred to as double mutation (DM), function to decrease fVIII binding to BiP, a resident ER chaperone, which results in increased fVIII secretion (Swaroop, Moussalli et al. 1997). Furthermore, addition of 6 N-linked glycosylation sites, designated 226/N6, located within the human B domain also increases human fVIII expression (Miao, Sirachainan et al. 2004). We previously demonstrated that porcine and certain hybrid human/porcine fVIII constructs are expressed at 10 – 14-fold greater levels than human fVIII (Doering, Healey et al. 2002; Doering, Healey et al. 2004). The aim of the current study was to directly compare various fVIII expression constructs in order to determine an optimal transgene for gene therapy applications. The following fVIII constructs were generated: human B-domain-deleted fVIII (HBDD-fVIII), HBDD-fVIII with a 14 amino acid linker between the A2 domain and the activation peptide (HSQ-fVIII), porcine fVIII containing a 24 amino acid linker (HEP-fVIII), hybrid human/porcine-fVIII which has porcine A1 and A3 domains (HP47), and modified HBDD, HSQ and HEP-fVIII constructs containing DM and/or 226/N6. Each construct was transiently transfected into BHK-M cells, and fVIII production between 48 – 72 hrs post-transfection was measured using a one-stage clotting assay. Under these conditions, the addition of the DM and 226/N6 significantly increased fVIII expression for HBDD (P = 0.003), though not for HSQ. Addition of DM or 226/N6 alone did not significantly increase the expression of either human fVIII construct, and furthermore, the addition of DM to HEP-fVIII decreased its expression 98%. HEP-fVIII was expressed at 8-fold or greater levels than any of the other human constructs. Next, ~25 stably transfected BHK-M clones were isolated following transfection with each of the fVIII expression constructs and the rate of fVIII production for each clone was determined. Several clones did not express detectable fVIII activity (<0.01 units/mL) and were excluded from the analysis. Approximately 14% of the total number of clones were excluded, ranging from 0 – 42% for the different constructs. HEP-DM-fVIII was the exception, where 82% of the clones had activity <0.01 units/mL. Mean HEP-fVIII expression was 3.93 ± 3.22 units/mL/24 hr (n = 19) (Figure 1), and HP47 was similarly expressed at 3.21 ± 2.31 units/mL/24 hr (n = 19). All of the HSQ-based constructs and HBDD-DM/226/N6 showed similar mean expression levels (0.28 ± 0.03 units/mL/24 hr) and were significantly higher than HBDD and HBDD-DM, which had a mean of 0.12 ± 0.01 units/mL. In the current study, we provide experimental evidence that the expression of HEP-fVIII and HP47 is superior to other bioengineered fVIII expression constructs, which should eliminate the expression barrier that has hampered the clinical translation of gene therapy for hemophilia A.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal