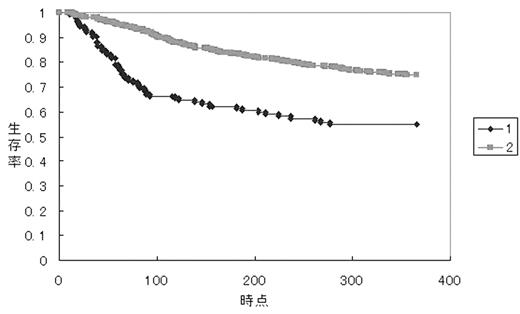
There is a growing demand for quality improvement in hematopoietic stem cell transplantation as in other fields of patient manegement. Indicators would be necessary for the quality control, which finally lead a continuoous quality improvement process. We analyzed the impact of 100-day survival on the outcome in leukemia patients receiving a myeloablative unrelated BMT at an early stage of leukemias. Data on 1,203 patients who received a myeloablative unrelated BMT at the first CR of acute leukemia or the first chronic phase of CML between 1993–2001 were retrieved from Japan Marrow Donor Program Registry’s database. There were 40 hospitals which performed more than 10 BMTs during this period. A significantly lower 100-day survival than the average of all patients (n=822) was found in six hospitals (group 1). The remaining 36 hospitals were grouped in group 2. Overall survival (OS) at 1-year was 0.56 in group 1 (n=125) and 0.75 in group 2 (n=697) (p<0.001).
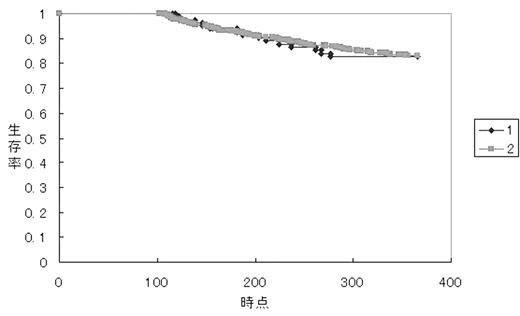
The 100-day survival was 0.66 (83/125) in group 1 and 0.90 (625/697) in group 2 (p<0.001). OS at 1-year for survivors over 100 days was 0.828 in group 1 and 0.832 in group 2 (p=0.9).
After adjustment for age (~15, 16~39, 40~), type of leukemia (CML, ALL, ANLL), and group (1, 2) by using the Cox regression model, patients in group 1 were found to have a higher risk of death than patients in group 2 (Hazard Ratio 2.14; 95% CI 1.58–2.90). These results indicate that
among hospitals which performed more than 10 BMTs between 1993–2003, 6 hospitals showed a significantly lower 100-day survival than the average of all patients,
OS at 1-year for survivors over 100 days in patients in these 6 hospitals was simillar in patients in the remainging 34 hospitals, and
after adjustment of age, type of leukemia and hospital group by using Cox regression model, patients in these 6 hospitals were found to have a higher risk of death than patients in the remaining 34 hospitals.
Thus, the 100-day survival might be an indicator for the quality control in leukemia patients who receive a myeloablative unrelated BMT at an early stage of leukemias.
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal