Abstract
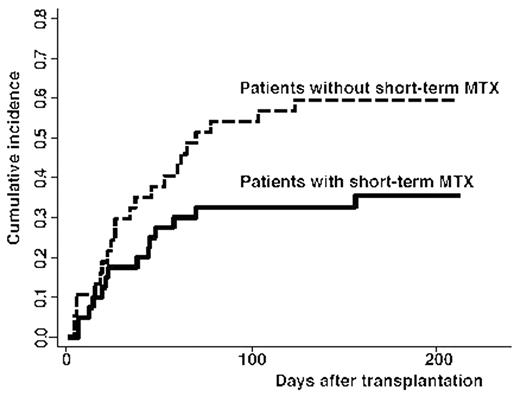
Cord blood transplantation (CBT) represents an attractive alternative for patients with advanced hematological malignancy who lack matched related or unrelated donors. Adult patients receiving myeloablative or reduced-intensity CBT display a 90% chance of engraftment, but also experience a 50% rate of transplant-related mortality. Post-transplant immune disorders including early immune reactions and acute GVHD are problematic in CBT for adult patients. These reactions and/or additional immunosuppressive therapy might increase the risk of infection and organ dysfunction, leading to high rates of transplantation-related mortality. However, optimal prophylaxis and management for immune reactions have not been established in CBT. We investigated retrospectively whether intensive graft-versus-host disease (GVHD) prophylaxis has a prognostic impact on CBT. Post-CBT immune reactions were classified according to time course as pre-engraftment immune reaction (PIR), engraftment syndrome (ES) or acute GVHD. Between March 2001 and September 2003, a total of 77 patients underwent CBT at 8 centers of the Nagoya Blood and Marrow Transplantation Group (NBMTG). Median age of patients was 48 years (range, 18–69 years). Preparative regimens were myeloablative (n=31) or reduced-intensity (n=46). Acute GVHD prophylaxis included cyclosporine alone (n=23), tacrolimus alone (n=12), cyclosporine plus short-term methotrexate (n=17), tacrolimus plus short-term MTX (n=23) or cyclosporine plus methylprednisolone (n=2). Cumulative incidences of PIR, ES and grade I-IV GVHD were 38%, 12% and 29%, respectively. Short-term MTX exerted significant favorable effects on PIR in univariate analysis (hazard ratio, 0.41; 95% confidence interval, 0.19–0.89) and on non-relapse mortality (NRM) in multivariate analysis (hazard ratio, 0.36; 95% confidence interval, 0.16–0.81). On the other hand, PIR occurred in 35% of patients with cyclosporine and 37% with tacrolimus. Short-term MTX appears to offer optimal GVHD prophylaxis to reduce PIR and improve NRM.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal