Abstract
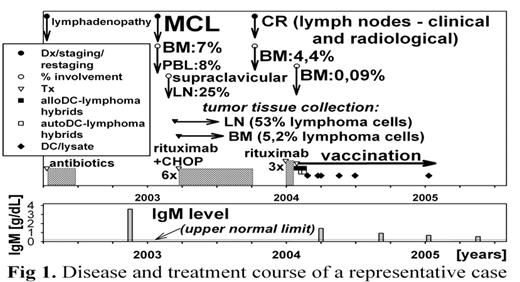
A feasibility of hybrid cell vaccination performed in remission following a standard chemotherapy (CHT) is currently being studied in patients with indolent B cell lymphomas at our institution under approval of the Ethics Committee. Adequate samples of fresh tumor tissue were collected from 16 patients. Seven patients were vaccinated with irradiated lymphoma/dendritic cell (DC) hybrids, and whenever the amount of collected viable lymphoma cells was not sufficient to complete vaccination with the use of cell hybrids, booster injections were alternatively performed with the use autologous DCs pulsed with tumor lysate. Autologous lymphoma cells were electrofused with autologous and/or allogeneic DCs, depending on accessibility of autologous DCs. Monocyte-derived DCs were basically generated from the peripheral blood adherent cells stimulated with GM-CSF and IL-4, and if available, from the bone marrow (BM) adherent cells. If only possible, autologous DCs were also generated from the BM non-adherent cells or from a sample of leukapheresis product collected for transplantation cultured with GM-CSF, TNF-α, SCF and FLT3-L to induce differentiation of DC progenitors. DC hybrids and/or DCs preincubated with tumor lysate were administered to uninvolved lymph nodes under USG guidance. Four patients failed to achieve a complete remission after initial therapy and the vaccination was discontinued due to disease progression (after 2, 2, 6, and 5 cycles). Three patients were vaccinated during remission following CHT. At present - 16, 18, and 10 months after starting the immunization, and after 8, 9, and after 7 cycles of vaccination, one patient with mantle cell lymphoma (MCL) is symptomless with persistent bone marrow (BM) involvement, one patient with MCL presenting with IgM paraproteinemia is disease-free with residual BM involvement (Fig 1), and one patient with follicular lymphoma is disease-free. Cutaneous delayed type hypersensitivity (DTH) response to DC/lymphoma hybrids exceeded the response to DCs alone in 3 of 7 vaccinated patients, and interferon-γ producing CD8+ cells specific to autologous lymphoma cells were demonstrated in one patient. In another patient, cutaneous DTH reaction was accompanied by transient pruritus and swelling of the injected lymph node two days after the sixth booster injection. No other adverse reactions to vaccinations were observed. Vaccination using the DC/lymphoma hybrids appears feasible, safe, and capable of inducing lymphoma cell specific immune responses. Further evaluation of efficacy in terms of disease control is mandatory.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal