Abstract
Objective: Rituximab was licensed (1997) to treat B-cell lymphomas. As a consequence of its B-cell depleting effect, Rituxmab has been widely used in autoimmune conditions including ITP. This study assessed the long term efficacy of Rituximab in patients with chronic ITP.
Methods: All patients with responses lasting more than 1 year (yr) to Rituximab treatment were included in this IRB approved study to determine the duration of response to Rituximab. Forty six patients fulfilled these criteria; thus far complete data are available for 31. The median follow up (f/u) was 2 3/4 yrs. At onset of Rituximab treatment, the 31 patients had platelet counts <30 x 10 9/l, had received two or more previous ITP treatments, and 14 (44%) had undergone splenectomy. The median age and duration of ITP were 32 yrs (range 12–65) and 1 3/4 yrs respectively. The patients received Rituximab at 375 mg/m2 weekly for 4 weeks. The 15 patients for which data are not available had similar characteristics.
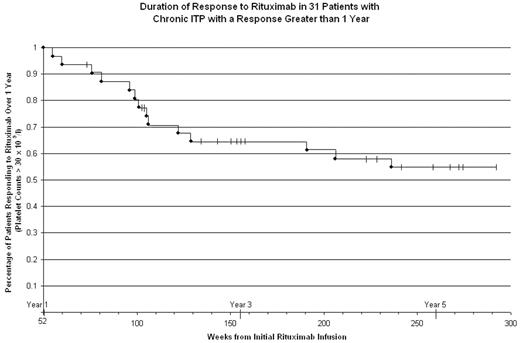
Results: The figure outlines what happens more than one yr from Rituximab treatment for those patients whose response lasted > 1 yr. Fourteen of the 31 patients have relapsed within the f/u period giving a 5 yr response rate of 55%. Eleven of the 14 relapsers did so within 2 1/2 yrs of their first infusion whereas 14 of the 17 patients with ongoing responses had f/u > 2 1/2 yrs. The data suggest that patients whose response is > 2 1/2 yrs have a low likelihood of relapse before 5 years. While duration of ITP prior to Rituximab treatment was significantly shorter (p< 0.001) for patients responding over 3 years (median=39 weeks) than it was for those responding between 1 and 3 years (median=176 weeks), no specific duration of ITP prior to which Rituximab should be instituted was evident. None of age, sex, time to a platelet count > 30 x 109/l, and splenectomy status predicted duration of response to Rituximab. Out of the original 31 responders, there were 25 complete responses (CR: platelet count >150 x 109/l) and 6 partial responses (PR: platelet count 30–150 x 109/l). Fifteen (60%) of the CRs and 2 of the PRs (33%) remain in lasting remission (p=NS). Characteristics such as median age, duration of ITP, gender, splenectomy status, and median duration of response were similar for both PRs and CRs. Data on toxicity are less well-developed; no serious infections, malignancies, or other major toxicities were seen in this group of patients. In conclusion, several studies including our own (Cooper Brit J Haem 2004) demonstrated that approximately 1/3 of Rituximab treated patients would have a duration of response lasting > 1 yr. Among this 1/3, now with considerable additional f/u, lasting responses to Rituximab were seen in more than half. Taken together, these findings imply that responses > 2 1/2 yrs in duration would occur in approximately 1/6 of the starting population of Rituximab-treated patients with chronic ITP with little further relapse in the subsequent 2 1/2 yrs. While informing the long term response to Rituximab, this study cannot yet provide a specific treatment algorithm.
Duration of Response to Rituximab in 31 Patients with Chronic ITP with a Response Greater than 1 Year
Duration of Response to Rituximab in 31 Patients with Chronic ITP with a Response Greater than 1 Year
Disclosures: Rituximab is not approved for the treatment of ITP.; Clinical research support from genentech for other studies.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal