Abstract
Waldenström Macroglobulinemia (WM) is characterized by monoclonal IgM paraprotein and bone marrow (BM) infiltration by lymphoplasmacytic lymphoma. The normal counterpart of WM malignant cell seems to be a post germinal centre IgM B-cell, which tumoral transformation occurs after cessation of somatic mutation (SM) but prior to Class switch recombination (CSR). However, recently has been reported that CSR can be possible “ex-vivo”, since clonotypic transcripts encoding post-switch isotypes have been observed in some WM cells cultured with CD40L/IL-40. However, this process has not been shown to occur “in vivo” until now.
#3754, a 51-year-old woman, was diagnosed in 2001 of WM with a M-IgM spike (46 g/L), anemia (Hb 9·6 g/dL), 76% lymphoplasmacytic monoclonal B-cells in BM and normal cytogenetics. In April 2005, an important IgG increase was observed (23 g/L). Immunofixation demonstrated an IgG-k paraprotein in the mid g-region and a monoclonal IgM-k paraprotein at the b-region corresponding to the two monoclonal peaks detected on serum electrophoresis. After 6-mercaptoethanol treatment, a single band was seen at the line stained with kappa, suggesting the presence of a single clone. Other causes of IgG monoclonal components were excluded considering clinical factors, immunophenotype analyses (San Miguel et al, 2003), quantity of DNA and cell cycle analyses (Ocio et al, 2005). However, the definitive proof for a unique monoclonal population was provided through molecular analysis. A single clonotypic rearrangement was detected by amplifying the complete VDJH fragment at diagnosis moment, according to the protocol describes in Biomed II (
In conclusion, we report for the first time a WM case in which tumor cells were able to carry out CSR, showing IgG and IgM clonotypic amplification, as well as producing both paraprotein components. This constitutes the first in vivo demonstration that CSR is possible in WM cells, and are able to develop a fully functional isotype class switch recombination not only in vitro but also in vivo.
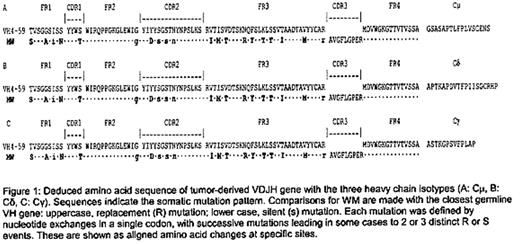
Deduced amino acid sequence of tumor-derived VDJH gene with the three heavy chain isotypes (A: Cμ, B: Cδ C: Cγ). Sequences indicates the somatic mulation pattern. Comparison for WM are made with the closest germline VH gone; uppercase, replacement (R) mutation; lower case, silent (S) mutation. Each mutation was defined by nuclieotide exchanges in a single codon, with successive mutations leading in some cases to 2 or 3 distinct R or S events. These are shown as aligned amino acid changes at specific sites.
Deduced amino acid sequence of tumor-derived VDJH gene with the three heavy chain isotypes (A: Cμ, B: Cδ C: Cγ). Sequences indicates the somatic mulation pattern. Comparison for WM are made with the closest germline VH gone; uppercase, replacement (R) mutation; lower case, silent (S) mutation. Each mutation was defined by nuclieotide exchanges in a single codon, with successive mutations leading in some cases to 2 or 3 distinct R or S events. These are shown as aligned amino acid changes at specific sites.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal