Abstract
Background. A photochemical treatment (PCT) using amotosalen HCl (S-59) and UVA light was developed to inactivate pathogens and leukocytes in therapeutic plasma (INTERCEPT™, I-FFP) frozen within 8 hr of collection. Previous studies demonstrated a broad spectrum of pathogen inactivation (
Aims. We measured coagulation factors in plasma isolated from whole blood held overnight at controlled temperature (21 ± 3°C), processed with pathogen inactivation, and frozen within 18 hr of blood collection.
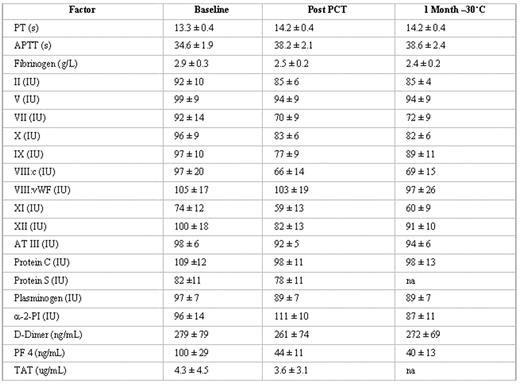
Methods. Whole blood units, approximately 460 mL, anticoagulated with CPD (Baxter, La Chatre, France) were drawn from group A, O, B and AB donors. Units were processed after 16 hr storage, and plasma was isolated by centrifugation. Two to 3 plasma units of matched blood group were pooled (n = 30: A = 14, O = 14, B = 1, AB =1) to a final volume of 635 mL. Baseline samples for assay of coagulation factors were withdrawn. Each of 30 pools was mixed with 15 mL of 6 mM amotosalen (150 uM: final concentration) and illuminated with a 3 J/cm2 UVA treatment. Following illumination (~ 8 min) and passage through a flow compound adsorption device (~20 min) to reduce levels of residual S-59, treated plasma units (650 mL) were divided into 3 equal storage units of ≥ 200 mL. Before freezing, post-treatment samples for assay of coagulation factors were withdrawn for assay of coagulation factors. Treated plasma units were flash frozen at -80°C, and transferred to −30°C for 12-month storage. Treated units were withdrawn after 1 month to measure total protein, albumin, IgG, IgM, IgA, fibrinogen, factors II, V, VII, VIII, IX, X, XI, XII, VIII-vWF, Proteins C and S, AT III, plasminogen, alpha-2 antiplasmin, D-dimers, PT, and APTT.
Results. Baseline coagulation factor levels (Mean ± SD) were in suitable therapeutic ranges. After PCT, all units had residual platelets < 1×109/L, WBC < 1×104/L, and RBC < 1 × 109/L. After PCT and frozen storage for 1 month, total protein (59 ± 2 g/L), albumin (38 ± 1 g/L), IgG (8.9 ± 1.1g/L), IgA (1.8 ± 0.4 g/L) and IgM (0.9 ± 0.3 g/L) were unchanged from baseline. Mean values for fibrinogen (g/L), coagulation factors (IU/dL), coagulation inhibitors (IU/dL), were variably reduced from baseline, but within ranges defined as suitable for therapeutic plasma (Table). There was no evidence of plasma activation.
Conclusions. Plasma prepared from whole blood after storage on cooling plates before processing with the INTERCEPT system for pathogen inactivation retained coagulation factor activity levels after frozen storage (−30°C) in conformance with French national standards for therapeutic frozen plasma (FP). Approximately 36 units (200 mL) could be prepared per hr of illumination time with this system.
Disclosures: Laurence Corash, Linda Pinlowski, and Yasmin Singh are employees of Cerus Corporation.; Laurence Corash, Linda Pinlowski, and Yasmin Singh own stock in Cerus Corp.; Jean Pierre Cazenave received research funding to support this study.; Jean Pierre Cazenave has served on a Science Advisory Board for Cerus Corp.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal