Abstract
Dendritic polymers are branched nanopolymers with a central core and multiple peripheral functional groups that offer great potential as high payload delivery vehicles carrying multiple copies of drug molecules, targeting ligands and imaging agents to their site of action. Their nanoscopic dimensions offer exciting possibilities for achieving high intracellular drug concentrations in many therapeutic areas including anti-cancer drug delivery. Biocompatibility and biodistribution of dendritic polymers may be influenced by surface charge and concentration. One of the major challenges in their use is the effect on coagulation. The objective of this study was to determine the effect of change in surface charge and concentration of dendritic polymer on cellular and enzymatic components of coagulation.
Materials and Methods: The effect of increasing concentrations (1, 10, 100, and 1000mcg/ml) of polyamidoamine (PAMAM) dendrimers with -COOH (anionic), -OH (neutral), and -NH2 (cationic) end functionalities, on platelet function and coagulation was evaluated using thromboelastography, whole blood aggregation, and flow cytometry. The thromboelastographic profile and platelet aggregation studies were obtained on samples of whole blood incubated for thirty minutes with dendrimer. Platelets were incubated with FITC labelled dendrimer for 30,60 and 120 mins, to determine uptake and platelet activation using flow cytometry. All tests were performed in triplicate.
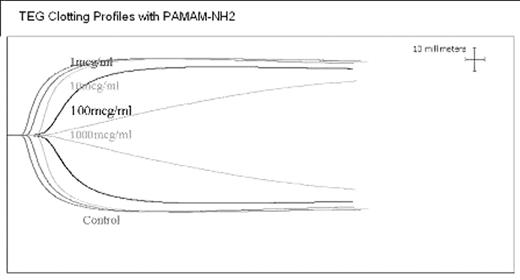
RESULTS: Thromboelastography: No significant effect on clot formation (time to clot formation and size) was seen with PAMAM-COOH (COOH) or PAMAM-OH (OH). Prolonged time to initiation of clot and decreased size were noted with 100 and 1000mcg/ml of PAMAM-NH2(NH2) as shown in figure1, indicating impairment of both the enzymatic and cellular components of the coagulation system. Whole Blood Aggregation: Neither platelet aggregation nor secretion were significantly affected by COOH or OH. Platelet aggregation was significantly decreased with NH2 at 100 and 1000mcg/ml. Flow Cytometry: Spontaneous CD62 activation was seen in platelets incubated with NH2. No spontaneous CD62 activation was noted with COOH or OH even at 1000mcg/ml. Platelet uptake of FITC labeled dendrimer was assessed at 30, 60 and 120mins of incubation. Increased uptake of FITC labeled dendrimer was noted at 2 hours with NH2.
TEG clotting Profiles with PAMAM-NH2.
CONCLUSIONS: Surface charge of the dendritic nanopolymers plays a significant role on its effect on coagulation and platelet function. The anionic -COOH terminated and neutral -OH terminated dendrimers had no effect on hemostasis even at the highest concentrations while the cationic-NH2 was associated with inhibition of platelet aggregation and delayed clot initiation at higher concentrations. This would indicate that the anionic and neutral dendrimers would serve as better vehicles than cationic dendrimers for targeted delivery of therapeutic agents.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal