Abstract
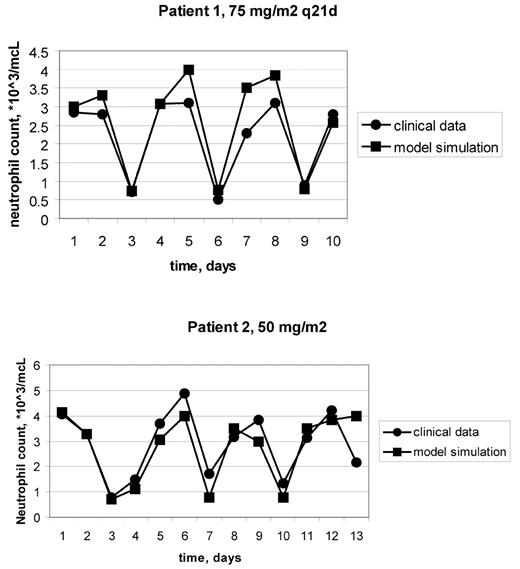
Neutropenia is a dose-limiting toxicity in dose-intensified chemotherapy regimens. Yet to be determined are the lower limit of inter-dosing interval of chemotherapy and the optimal schedules of GCSF support. In the absence of better tools, the most promising schedules to be tested in clinical trials are selected by trial and error. In order to provide a scientific tool for treatment selection, a physiologically-based, computer-implemented, mathematical model of human granulopoiesis was recently developed (Vainstein et al, J Theor Biol, 2005). The aim of the current study is to validate the model clinically and to use it for suggesting an improved doxorubicin monotherapy schedule, with GCSF support. First, the model was validated by showing its accurate predictions of neutropenia dynamics in patients treated by doxorubicin 75mg/m2 q14d, with GCSF support (clinical data from Bronchud et al, Br J Cancer, 1989). To validate the model ability to predict treatment outcomes for individual patients, it was simulated in conjunction with base-line blood counts and treatment schedules of ten breast cancer patients, who received different doxorubicin monotherapy protocols (20–30mg/m2 q7d, 60–75mg/m2 q21d). Model predictions for each patient were then compared with the patient’s neutrophil profile. Results showed that the model accurately predicted doxorubicin-induced neutropenia course in all patients examined (see example in Figure 1). To identify a new intensified regimen, which minimizes myelotoxicity, simulations were performed of different doxorubicin+GCSF treatment schedules. Results suggest that 4 days is the optimal gap before starting GCSF support, following administration of doxorubicin 75mg/m2 q14d. No grade 3/4 neutropenia is expected under such a regimen, as compared to 3–4 days neutropenia when the gap before starting GCSF support was 1 day (Bronchud et al, 1989). It is further predicted that under the suggested schedule of GCSF support, doxorubicin monotherapy can be intensified, either to120mg/m2 q14d, by dose-escalation alone, or to 90mg/m2 q10d, by combining dose-escalation and increased dose-density. Such intensification is expected to result in 1–2 days of grade 3/4 neutropenia, as compared to 3–4 days long neutropenia in the doxorubicin schedule used by Bronchud et al., which has only 62% of the proposed doxorubicin dose intensity. Further research is warranted for clinically validating the superiority of the suggested treatment schedules. These results suggest that if the involved cellular dynamics are precisely calculated, doxorubicin schedule can be further intensified, accompanied by GCSF support, with no significant increase in myelotoxicity. Showing precision in predicting individual patient’s counts, the computer model of human granulopoiesis can be used for treatment personalization.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal