Abstract
^Renal Safety Board (RSB) member
Introduction: Deferasirox has been evaluated in a series of 1-year clinical trials in patients with a variety of transfusion-dependent anemias. Mild, non-progressive increases (ie initial rises, then stabilization) in serum creatinine >33% above baseline were observed in approximately one-third of patients receiving deferasirox. Following completion of the core trials, patients have entered 4-year extension phases to evaluate the long-term safety and efficacy of deferasirox. This is an analysis of renal function in patients receiving deferasirox in two ongoing extension phases.
Methods: Initial deferasirox dose was assigned by baseline iron burden in the core trials, and tailored to meet individual patient needs in the extension phases. Serum creatinine and urinary protein/creatinine ratios were measured monthly. Creatinine increases >33% above baseline on ≥2 consecutive values, ≥7 days apart, were selected by the RSB for dose review.
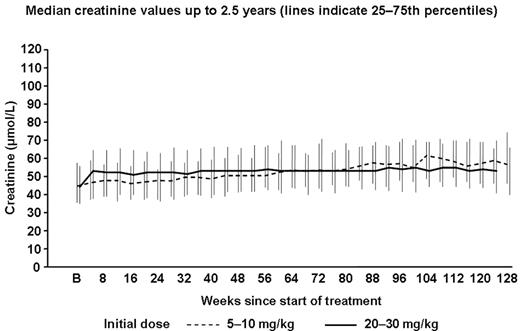
Results: Of the 480 patients who initially received deferasirox, 387 entered the extension phases and 334 were on treatment at the time of analysis; the median duration of treatment was 2.5 years. In patients who started on deferasirox 20 and 30 mg/kg/day, non-progressive, dose- and iron intake-dependent creatinine increases were noted by week 4 (Figure). In patients who initially received deferasirox 5 and 10 mg/kg/day, a small, non-progressive increase also occurred near week 52, coinciding with dose escalation to 20 or 30 mg/kg/day. These patterns were consistent irrespective of age or underlying anemia. Similar trends were observed with creatinine clearance. Of the 480 patients, creatinine levels increased marginally above the upper limit of normal (ULN) at some point during the 2.5-year observation period in 12.1%. With or without dose reduction, most (72.4%) returned <ULN, 5% fluctuated around ULN and 15.5% stabilized slightly >ULN. No follow-up information was available in 7.1% of patients at the time of data cut-off.
Median creatinine values up to 2.5 years (lines indicate 25-75th percentiles)
Median creatinine values up to 2.5 years (lines indicate 25-75th percentiles)
These non-progressive creatinine increases were generally reversible with dose modification/interruption. In 36 episodes of treatment interruption, preceded by creatinine increases >33% above baseline, overall median creatinine levels decreased from 77.8 to 62.8 μmol/L. Levels fell below the <33% limit in 25 episodes, and decreased rapidly but remained >33% above baseline in the remaining 11, where treatment was either resumed early or the patient was off-treatment at the time of analysis. Ten patients (1.0%) had urinary total protein/creatinine ratio >1.0 at two consecutive visits.
Conclusions: This 2.5-year analysis of renal function in patients receiving deferasirox confirms that early changes in creatinine/creatinine clearance observed in 1-year core trials are non-progressive with continued treatment. The small changes in creatinine/creatinine clearance noted when patients were dose-escalated from 5/10 mg/kg/day at the end of the core trials, were also non-progressive. These changes were manageable with appropriate dose modification.
Disclosures: E Glimm and J Ford are employees of Novartis Pharma AG.; C Ponticelli - external consultant of Novartis Italy for organ transplantation, autoimmune diseases.; A Piga - Novartis, Apotex and Genzyme for studies on iron chelators.; A Kattamis - honoraria received.; A Piga - Advisory committee membership for studies on iron chelators sponsored by Novartis and Apotex; A Kattamis - Participation on the Exjade speakers’ bureau.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal