Abstract
One-third of adults with sickle cell disease (SCD) have echocardiographic (ECHO) evidence of pulmonary hypertension (PHTN), a complication associated with early mortality. Because the pulmonary artery pressures in most SCD patients with PHTN are only mildly elevated, the question arises whether such increases are primarily a reflection of the high cardiac output state that accompanies the anemia.
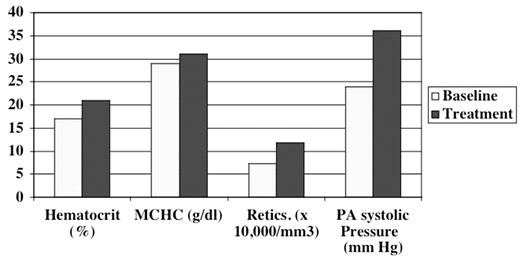
Recently we treated a 45 year-old woman with homozygous sickle-cell disease and profound iron deficiency due to heavy menstrual flow. Two ECHOs were done while she was severely iron deficient (Hb 5 g/dl, MCV 57 fl, reticulocytes 72,407/mm3, serum bilirubin 0.5 mg/dl, iron 29 mcg/dl, transferrin 376 mg/dl, and ferritin 3.6 ng/ml). Her pulmonary artery systolic pressure (PAs) was calculated from the tricuspid regurgitant jet velocity (TRV) using the Bernoullie equation: 4(TRV3) + central venous pressure (assumed at 10 mm Hg). The PAs was normal, 24 mm Hg, even though the patient also had M-mode evidence of left ventricular diastolic dysfunction and a small pericardial effusion. Treatment with intravenous iron and red cell transfusion partially improved her iron deficiency and anemia (Hb 7 g/dl, MCV 67 fl, serum bilirubin 0.7 mg/dl, iron 54 mcg/dl, transferrin 322 mg/dl, and ferritin 33.9 ng/ml) but also increased her hemolytic rate: though LDH data are unavailable, the reticulocyte count rose to 117,900/mm3. Repeat ECHO exams at this time showed that her pulmonary artery systolic pressures increased to 35–36 mm Hg. These values are at or near the lower range of pulmonary artery systolic pressures (36–70 mm Hg) measured in SCD patients in whom PHTN was diagnosed at cardiac catheterization. The figure compares hematologic values, and pulmonary artery systolic pressure in our iron deficient SS patient at baseline and during treatment.
This experience, though anecdotal, suggests that the PHTN in SCD is unrelated to the anemia per se and, by implication, also unrelated to the high cardiac output. The patient’s mild pulmonary systolic hypertension actually developed with improvement of her anemia. Our hypothesis is that when the patient’s iron deficiency was most severe, the low MCHC decreased Hb S polymerization and decreased hemolysis, as in other iron deficient SCD patients. Her relatively low hemolytic rate may have prevented the mild PHTN, which developed once treatment improved her iron deficiency but increased hemolysis. Our hypothesis is consistent also with an emerging new paradigm in sickle cell disease pathophysiology: a strong link between hemolysis-related nitric oxide system (NO) dysfunction and risks for pulmonary hypertension, leg ulcers, priapism, and death. In this context it is interesting that iron deficiency anemia up-regulates vascular nitric oxide synthase in animals. In humans iron deficiency increases NO production even in the absence of anemia. Hence, this patient’s iron depletion may have contributed to the maintenance of her low pulmonary pressures also by a direct NO-mediated vascular effect.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal