Abstract
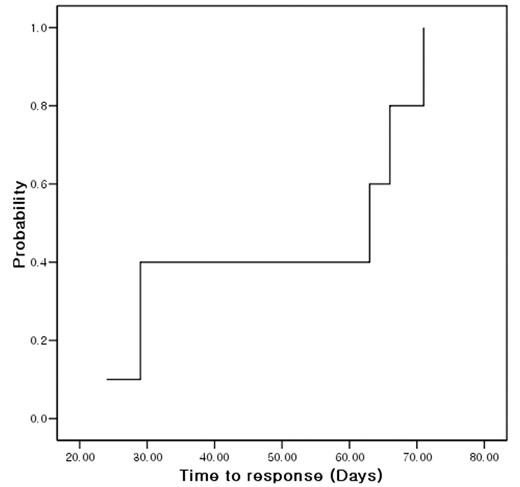
Patients with bone marrow failure syndrome are suffered from cytopenia and resulting requirement of transfusion. Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the standard therapy for severe aplastic anemia (AA) with suitable sibling donor. However, it is true in many cases that alloHSCT can not be performed even in very severe AA due to no suitable donor. In that regards, immunosuppressive therapy (IST) is another option for those patients. Alemtuzumab (ALM) targets not only B-cell but also T-cell, which is known to play an important role in pathogenesis of bone marrow failure syndrome (BMFS). Although there were several reports of ALM for immune cytopenia and pure red cell aplasia, no report were published regarding ALM for BMFS. We studied the feasibility of ALM and cyclosporine A (CsA) for patients with BMFS. Inclusion criteria were transfusion-dependent BMFS including AA, MDS, PNH. Prior IST or other drugs for BMFS except for ALM were not excluded. ALM was infused for 3 days (10mg on day 1, 20mg on day 2, and 30mg on day 3) along with CsA for at least 6 months. Prophylactic ciprofloxacin, acyclovir, and bactrim were given for first 2 months. Without partial response (PR) or more within 6 months, subsequent alloHSCT or antithymoglobulin therapy was allowed. Total 12 patients were enrolled. Female were 9 (75%). Median age was 43 (16–74) years. Disease were 9 severe AA, 2 moderate AA and 1 hypoplastic MDS. All patients were transfusion-dependent. Median amount of transfused red cell, platelet concentrate and platelet apheresis prior to ALM-CsA were 2, 2 and 7 units, respectively. Prior therapy were none in 9 (75%), anti-thymoglobulin in 2 (16.7%), and danazol in 1 (8.3%). Only 1 patient could not receive full scheduled CsA because the patient underwent alloHSCT. Median follow-up was 326 (140–466) days. Response to ALM-CsA were no response in 7 (58.3%), PR in 4 (33.3%), and CR in 1 (8.3%). Therefore, overall response rate was 5/12 (41.7%). All responsive patients showed their responses within 3 months. Median time to response was 47 (24–71) days [Figure 1]. PR was converted to CR in 1 patient at 12 months after treatment. Neither fever nor skin rash during treatment did not affect the possibility of response to ALM-CsA (p=0.523, p=0.523, respectively). Toxicity during ALM was fever in 3 (25%), skin rash in 3 (25%), G1 ALT elevation in 7, G1 AST elevation in 4, and hyperbilirubinemia in 3 (G1 in 2, G3 in 1). There were no anaphylaxis, serum sickness or CMV reactivation.
In spite of small number, AML-CsA showed comparable response rate within 3 months after treatment. Therefore in conclusion, ALM-CsA is feasible and tolerable therapy for BMFS.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal