Abstract
Jak2 is a cytoplasmic tyrosine kinase that has been linked to hematological malignancies. Recently, a somatic Jak2 mutation (Jak2-V617F) has been identified in several myeloproliferative disorders such as polycythemia vera, essential thrombocythemia and myeloid metaplasia with myelofibrosis. These myeloproliferative disorders are characterized by unregulated expansion of one or more cells in the blood. The presence of the Jak2-V617F mutation in cells results in uncontrolled cell division and resistance to the negative feedback mechanisms that govern normal cell growth. The availability of a Jak2 tyrosine kinase specific inhibitor would facilitate our understanding of these Jak2-related disorders and perhaps serve a clinical benefit for patients. However, the most widely used Jak2 inhibitor, AG490, suffers from a general lack of specificity. Here, we used a novel approach to identify Jak2-specific inhibitors. We used in silico homology modeling of the Jak2 kinase domain to identify solvent accessible exposed pockets on the surface of the Jak2 protein. We then used a high-throughput program called DOCK to predict the ability of 20,000 small molecules to interact with a structural pocket adjacent to the ATP binding site of murine Jak2. The predicted binding energies of interaction between each compound and the Jak2 kinase domain were calculated and the top six scoring compounds were tested for their ability to inhibit Jak2 tyrosine kinase function in vitro. One of these compounds, 2-methyl-1-phenyl-4-pyridin-2-yl-2-(2-pyridin-2-ylethyl)butan-1-one (Z3) effectively inhibited Jak2-V617F and Jak2-WT autophosphorylation. We were able to show that Z3 inhibits total Jak2 tyrosine phosphorylation as well as Jak2 phosphorylation at the critical tyrosine 1007 residue in both a dose-and time-dependent manner. Z3 is able to reduce growth hormone-dependent Jak2 activation and is a direct inhibitor of Jak2-WT and Jak2-V617F tyrosine kinase autophosphorylation as measured by an in vitro kinase assay. Moreover, we found that Z3 inhibits proliferation of human erythroleukemia (HEL) cells, which express the Jak2-V617F mutation. In summary, this work demonstrates proof-of-principle concept that in silico molecular modeling can be used as a means to identify specific tyrosine kinase inhibitors.
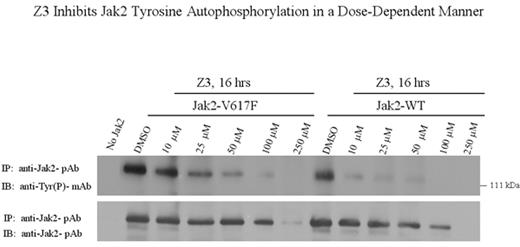
Z3 Inhibits Jak2 Tyrosine Autophosphorylation in a Dose-Dependent Manner
Z3 Inhibits Jak2 Tyrosine Autophosphorylation in a Dose-Dependent Manner
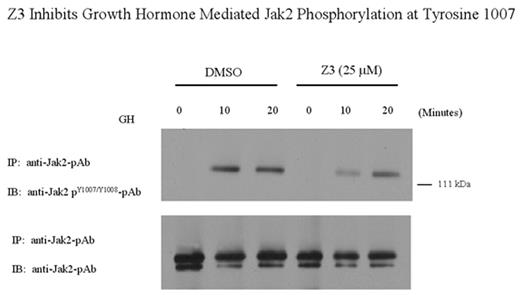
Z3 Inhibits Growth Hormone Mediated Jak2 Phosphorylation at Tyrosine 1007
Z3 Inhibits Growth Hormone Mediated Jak2 Phosphorylation at Tyrosine 1007
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal