Abstract
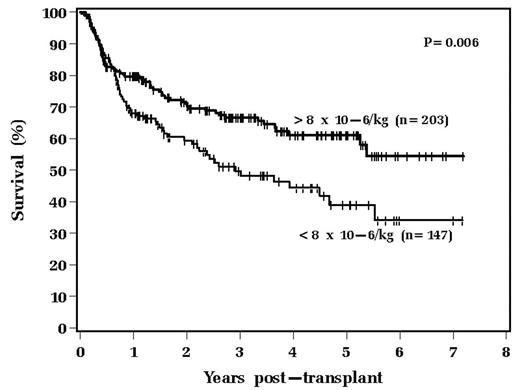
We have anecdotally observed that many patients mobilized with Etoposide (VP) + filgrastim (G) collect very high numbers of CD34+ cells for autologous stem cell transplantation (ASCT). Some BMT centers have suggested that the cellular composition of an autologous graft may influence ASCT outcome. We therefore queried whether patients collecting high numbers of PBPCs (“super-mobilizers”) have a better outcome than other patients. We retrospectively reviewed 693 consecutive adult patients with Non-Hodgkin Lymphoma (NHL) or Hodgkin Lymphoma (HL) receiving an ASCT from 1/1994 through 12/2005 treated with a uniform preparative regimen of Busulfan, Cyclophosphamide and Etoposide (Bu/Cy/VP). Of these 693 patients, 350 were mobilized with VP (2 gm/m2) + G and comprised the study population. After receiving VP+G, patients were collected for a minimum of 2 days with a collection goal of 7 × 106 CD34+ cells/kg. A minimum collection of 2.0 × 106 cells was required to proceed to ASCT. Super-mobilizers were defined as collecting, and infusing, greater than 8 × 106 CD34+ cells/kg. 203 patients were super-mobilizers, while 147 collected between 2.0 and 7.95 CD34+ cells/kg. Pre-transplant performance status, LVEF, and DLCO were similar between the two groups. Super-mobilizers were slightly younger (mean 47 years old vs. 51 years old, p=0.003) and more likely to have received 2 or fewer prior chemotherapy regimens, (80% vs. 63%, p<0.001). 82% of the entire study population had NHL and 18% HL, and 83% had chemotherapy sensitive disease at the time of ASCT. The super-mobilizer group required fewer days of pheresis for mobilization (median 2 days vs. 5 days, p<0.001). The median CD34+ dose of the super-mobilizer group was 13.7 × 106/kg vs. 4.4 ×106/kg in the standard collecting group. The super-mobilizer group had a superior overall survival as shown graphically below:
A multivariable analysis was performed, and revealed three variables associated with improved survival: disease status at transplant (comparing patients in remission vs. those with refractory disease, p=0.004), younger age at transplant (p=0.033), and CD34+ cell dose (>8 vs. <8, p=0.038). We conclude that patients mobilized with VP+G achieving robust CD34+ cellular mobilization have an improved outcome after ASCT. The mechanism of action of this phenomenon is conjectural; further studies are required to determine if the composition of the infused graft directly influences outcome, and, if so, what specific cellular components are important. However, collecting large numbers of CD34+ cells may be clinically beneficial (regardless of the specific mechanism of action) and may warrant a change in definition of collection end points.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal