Abstract
We previously demonstrated early safety and efficacy of fixed-dose IV BuFlu. Here we compare IV BuFlu and IV BuCy2, with long follow-up in a large number of pts. Since pts were treated on consecutive, not randomized programs, we used pts receiving Flu-Melphalan (M) in a program spanning the time of the IV Bu-based studies to estimate/correct for this non-randomized “period” effect.
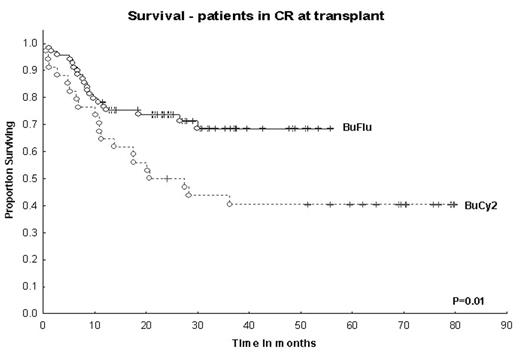
Methods: 293 pts received Bu 130 mg/m2, Flu 40 mg/m2 (after April 2001; n=148); Bu 0.8 mg/kg × 16, Cy 60 mg/kg × 2 (before April 2001; n=67); Flu 30mg/m2 × 4, M 140–180 mg/m2 (FM; before and after April 2001; n=78). Patients received FM mainly based on higher age and coorbidities. Graft-vs-host dx (GVHD) prophylaxis was tacrolimus-based. Bayesian log-normal regression models were used to assess covariate- and treatment effects on survival, non-relapse mortality (NRM) and time to progressive disease (TPD). Covariates and survival model are shown in the table. The model accounts for the “period” effect, the effects of Bu-vs-FM, and Flu-vs-Cy within IV Bu pts. Patient and treatment characteristics were (BuFlu, BuCy2 and FM): median age 46 (19–66), 39 (13–64) and 54 (22–74) years. At HSCT 47%, 48% and 21% of the patients were in CR1-3. Donors were HLA-identical siblings in 53%, 79% and 41%. 30% of the Bu groups and 47% of FM had poor prognosis cytogenetics. Results: Median follow-up in months (BuFlu, BuCy2 and FM; alive pts): 30 (9–56; n=80; all but 2 pts followed for at least 1 yr), 69 (24–102; n=23) and 48 (17–89, n=25). 100-day NRM for pts in CR at HSCT was 2% and 9% after Bu-Flu and BuCy2, while 1 yr-NRM was 7% and 18%. BuFlu produced significantly better EFS (P=0.04) and overall NRM (P=0.02) than BuCy2, but TPD was similar (P=0.34). 2-yr actuarial survival (CR pts): 75% (BuFlu) vs. 50% (BuCy), P=0.01.
Conclusion: After accounting for covariate and treatment period effects, there was a significant benefit to being treated with BuFlu compared to BuCy2 in spite of a higher median age and proportion of MUDs. The benefit was most evident for CR-pts due to a significant reduction in NRM, without loss of antileukemic activity due to exchanging Cy with Flu.
Fitted Bayesian log-normal overall survival model
| Variable . | Mean . | SD . | Posterior 95% credible interval 2.5% 97.5%* . | Probability of beneficial effect . | |
|---|---|---|---|---|---|
| *1=beneficial effect; 0=low likelihood of beneficial effect | |||||
| Cytogenetics | −0.85 | 0.4 | −1.652 | −0.045 | 0.017 |
| Disease status (CR vs other) | 1.12 | 0.55 | 0.019 | 2.15 | 0.975 |
| Donor (sibling vs other) | 0.606 | 0.237 | 0.149 | 1.056 | 0.992 |
| Age | −0.022 | 0.010 | −0.041 | −0.003 | 0.017 |
| Circulating blasts (0 vs >0) | 0.951 | 0.268 | 0.454 | 1.463 | 1 |
| Platelet count | 0.002 | 0.001 | 0.000 | 0.004 | 0.967 |
| Period effect | −0.914 | 0.458 | −1.795 | 0.041 | 0.029 |
| IV Bu (vs FM) | −0.513 | 0.481 | −1.455 | 0.420 | 0.148 |
| BuFlu (vs Bucy2) | 1.476 | 0.544 | 0.406 | 2.555 | 0.995 |
| Variable . | Mean . | SD . | Posterior 95% credible interval 2.5% 97.5%* . | Probability of beneficial effect . | |
|---|---|---|---|---|---|
| *1=beneficial effect; 0=low likelihood of beneficial effect | |||||
| Cytogenetics | −0.85 | 0.4 | −1.652 | −0.045 | 0.017 |
| Disease status (CR vs other) | 1.12 | 0.55 | 0.019 | 2.15 | 0.975 |
| Donor (sibling vs other) | 0.606 | 0.237 | 0.149 | 1.056 | 0.992 |
| Age | −0.022 | 0.010 | −0.041 | −0.003 | 0.017 |
| Circulating blasts (0 vs >0) | 0.951 | 0.268 | 0.454 | 1.463 | 1 |
| Platelet count | 0.002 | 0.001 | 0.000 | 0.004 | 0.967 |
| Period effect | −0.914 | 0.458 | −1.795 | 0.041 | 0.029 |
| IV Bu (vs FM) | −0.513 | 0.481 | −1.455 | 0.420 | 0.148 |
| BuFlu (vs Bucy2) | 1.476 | 0.544 | 0.406 | 2.555 | 0.995 |
Survival – patients in CR at transplant
Disclosures: IV busulfan for allogeneic transplantation in combination to fludarabine.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal