Abstract
Background: We previously reported that 20 patients with newly diagnosed, stage III/IV mantle cell lymphoma (MCL) treated prospectively with an in vivo rituximab purge, autologous stem-cell transplantation (ASCT) and rituximab maintenance experienced superior progression free survival (PFS) compared to 40 matched, historical controls treated with conventional chemotherapy. We now report extended follow-up from this trial.
Methods: Eligible patients were treated with 4–6 cycles of CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) followed by high dose therapy (cyclophosphamide, carmustine and etoposide) and ASCT. Rituximab (375 mg/m2) was given once 5 days prior to stem cell collection (as an in vivo purge), and as two, 4-week maintenance courses 8 and 24 weeks after ASCT. Historical control patients matched for age, stage and gender were randomly selected from a lymphoma database maintained by the British Columbia Cancer Agency. Two control-patients were selected for every trial patient. All control patients were treated with either an anthracycline based-regimen, or a regimen containing fludarabine and cyclophosphamide.
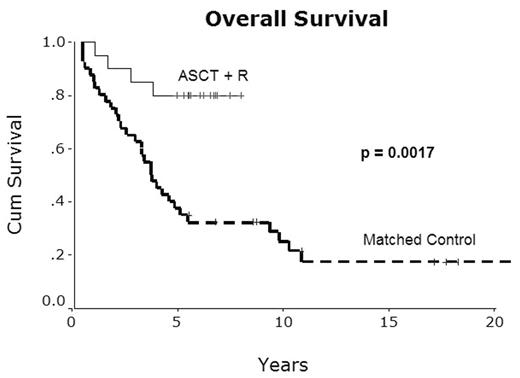
Results: Median follow-up is 5.3 years for the experimental cohort and 10.1 years for the control cohort. In the ASCT cohort, 8 patients have relapsed and 4 patients have died, 2 of progressive disease, 1 of a presumed cardiac event 7 months post-ASCT, and one of hepatitis B reactivation 11 months post-ASCT. Five-year OS and 5-year PFS were superior in MCL patients treated with ASCT+ rituximab compared to matched, historical MCL patients treated with conventional chemotherapy (5y OS 80% vs. 38%, P=0.0017; 5y PFS 72% vs. 19%, P=0.0001). One patient developed myelodysplastic syndrome 5.5 years after ASCT, and one patient was diagnosed with breast cancer 4.8 years after ASCT.
Conclusions: With more than 5 years of follow-up MCL patients treated with ASCT plus rituximab continue to experience significantly improved OS and PFS compared to matched, historical controls.
Overall Survival
Disclosures: Dr. Berinstein owns stock in Genentech.; This study was partially funded by Genentech Inc. Dr. Connors, Imrie, Berinstein and Buckstein conduct research supported by Roche Canada and/or Hoffman/LaRoche.; Dr.’s Berinstein, Buckstein and Imrie have received honoraria from Roche Canada.; Dr.’s Imrie and Berinstein have served on an Advisory Board for Hoffman/La Roche.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal