Abstract
The potential benefit of RIC allo-SCT in MM is still controversial. This single centre study aimed to evaluate the role of RIC allo-SCT for relapsed MM, using a genetic randomization through a “donor” vs. “no donor” comparison. Between 2002 and 2005, 32 consecutive patients with relapsed or refractory MM, and with an identified sibling, were referred to our centre for HLA typing, because they were considered as potential candidates for RIC allo-SCT. In all, 19 patients (59%; donor group) had an HLA-identical sibling donor, while the remaining 13 patients (41%; no donor group) had no HLA-identical sibling donor. There were no significant differences between these two groups that were comparable as for demographic features, disease characteristics and prognosis risk factors. Median age was 54 (range, 37–65), and all patient had previously failed at least one autologous SCT. Patients from the “no donor” group received salvage therapy including thalidomide, bortezomib, dexamethasone, and/or additional high dose chemotherapy.
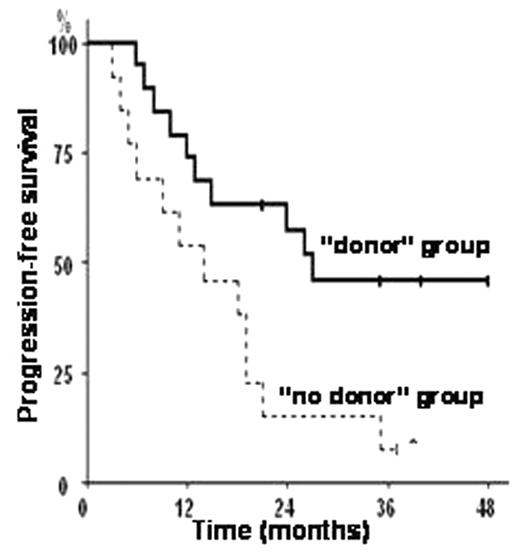
Among the 19 patients from the “donor group”, 18 (95%) could proceed to RIC allo-SCT. Hematopoietic recovery (ANC>500/μL and platelets >20000/μL) was achieved in all patients at a median of 16 (range, 0–28) and 5 (range, 5–30) days respectively. With a median overall follow-up of 36 months, 11 patients (85%, 95%CI: 54–98%) from the “no donor” group had disease progression despite salvage therapy, and only 6 of them are still alive, of whom 5 (80%) in progressive disease at last follow-up. In sharp contrast, only 5 patients (28%; P=0.001) from the “donor” group progressed after RIC allo-SCT. With a median follow-up of 49 months in the RIC allo-SCT group, 10 patients (56%; 95%CI; 33–79%) are still alive, with 4 patients being in complete remission, and 5 in partial or very good partial remission. Only one patient is currently experiencing disease progression and receiving additional salvage therapy. Interestingly, 11 patients (61%; 95%CI, 39–83%) from the RIC allo-SCT group showed objective disease response, usually concurrent to chronic GVHD. In all, 6 patients died from TRM for an overall incidence of TRM of 33% (95%CI, 11–55%) in this population of heavily pre-treated and relapsed or refractory MM population. In an intention-to-treat analysis, the KM estimate of progression-free survival was significantly higher in the “donor” group as compared to the “no donor” group (P= 0.01; 46% vs. 8% at 3 years; figure below). In all, these results compare favorably with those achieved using other standard non-allo-SCT salvage therapies for refractory or relapsed MM. Therefore, RIC allo-SCT from an HLA-identical sibling is a feasible and potential therapy that should be proposed for refractory or relapsed MM, since a potent graft-vs.-MM effect can be induced despite heavy pre-treatments, allowing for significantly longer PFS. Also, the latter results are expected to be further improved with the systematic and early use of maintenance therapies after RIC allo-SCT.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal