Abstract
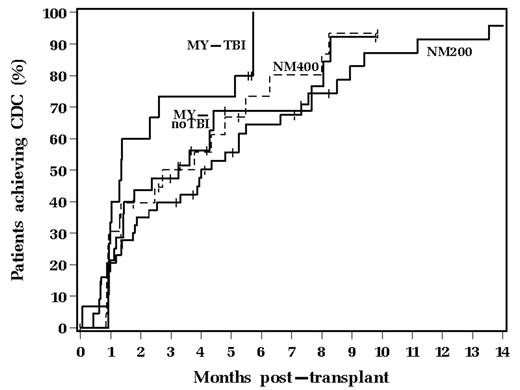
Assessment of chimerism after AHSCT has been important to monitor donor hematopoietic engraftment and to assess for residual disease or relapse. Achievement of T cell (CD3+) complete donor chimerism (CDC), particularly after NM AHSCT, has been considered important to achieve a graft-vs.-malignancy effect. Formal comparisons of the onset and frequency of T cell CDC in pts treated with MY vs. NM conditioning regimens have not been well described. The current analysis compared rates of achieving T cell CDC for 116 pts transplanted from 1/10/00–5/19/06 who were categorized into the following 4 groups based upon type of transplant conditioning: 1) 200 cGy total body irradiation (TBI) + fludarabine 30 mg/m2/d × 3 days (NM200; n=47); 2) 400 cGy TBI + fludarabine (NM400; n=23); 3) MY AHSCT with busulfan/cyclophosphamide without TBI (MY-noTBI; n=31); 4) MY AHSCT with TBI (MY-TBI; n=15). All NM AHSCT pts received peripheral blood stem cells and all MY AHSCT patients received bone marrow as their stem cell source. The total nucleated cell (TNC) and CD34+ cell doses were higher in the NM AHSCT patients. 36 (31%) pts had matched unrelated donors, all with at least an 8/8 match (HLA-A, -B, -Cw, -DR) by HLA class I and II DNA-based typing [10 (21%) NM200, 7 (30%) NM400, 9 (29%) MY-noTBI, 10 (67%) MY-TBI; p=0.011]. Graft-vs-host disease prophylaxis consisted of mycophenolate mofetil and cyclosporine or tacrolimus for most pts. Short tandem repeat analysis for T cell (CD3+) chimerism was performed on peripheral blood post transplant and CDC was defined as achievement of >95% DNA of donor origin isolated from CD3+ T cells. Post-transplant T cell chimerism was found in both the MY and NM AHSCT groups. The number of pts who achieved CDC and the median time to its occurrence for each group was as follows: NM200 - 34 (72%) at 4 mos; NM400 - 18 (78%) at 2.7 mos; MY-noTBI - 20 (65%) at 3.3 mos; and MY-TBI - 13 (87%) at 1.3 mos.
The Kaplan-Meier curves for achievement of CDC are shown above (p=0.23). The group of pts who received MY-TBI developed T cell CDC more rapidly than the NM200 pts (p=0.05). No significant differences were observed between the NM400, MY-noTBI and MY-TBI groups with regards to achieving CDC. NM conditioning with either 200 cGy or 400 cGy TBI did not show a significant difference in rate of achieving T cell CDC as compared to MY conditioning with busulfan/cyclophosphamide without TBI. This may be related in part to the higher TNC and CD34+ cell doses in the NM AHSCT pts. However, the increased TBI doses utilized for MY conditioning may more effectively suppress anti-donor immune effector cells from the recipient, which resulted in the increased CDC compared to the NM200 group. In conclusion, post-transplant monitoring for T cell CDC is important in both MY and NM AHSCT to allow for immune manipulation to maintain a state of donor-host tolerance in order to prevent graft rejection.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal