Abstract
A new and improved method for measurement of minimal residual disease (MRD) was developed.
Method
the total repertoire of leukaemic rearrangements of the immunoglobulin heavy chain (IgH) gene.was determined by performing multiple parallel Q-PCRs in microplates to determine usage of individual V and J segments. This enabled detection and quantification of clones ranging in size from 100% to approximately 0.03% of the leukemic population.
MRD measurement involved nested Q-PCR using the specific V and J primers and internal primers based on the sequence of the rearrangement of interest.
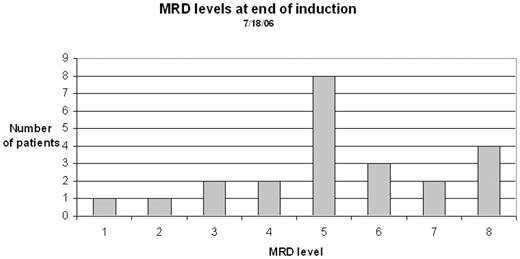
Following informed parental consent, 25 children with B-ALL were studied at the end of induction therapy. Under general anesthesia, 4 aspirations, 2 from each iliac spine, were performed and on each sample MRD was measured on 2 different days.
This enabled definition of the laboratory and sampling factors important in measurement of MRD.
Results
Repertoire analysis was very effective in identifying rearrangements of the IgH gene suitable for use as molecular markers, being superior to the commonly-used BIOMED-2 protocol for identification of both large and small clones. Two or more rearrangements marking large clones were detected in 20 of the 25 patients.
The median MRD level at the end of induction was 2.1 x 10−5. A level of > 10−3 was seen in 4 patients and < 10−7 in 4. Sensitivity of detection in a single sample was approximately 2 x 10−6, which is 1–2 orders of magnitude better than current techniques.
The SD of measurement depended on the number of target rearrangements present in the sample, reflecting the Poisson statistical uncertainty inherent in measurement of small numbers of events. For > 50 rearrangements SD was 0.23 log units, but below this level the SD rose steeply. 50 targets in 1 μg of DNA corresponds to an MRD of approximately 3 x 10−4.
There was significant sampling error. In 1 patient there was 1000-fold MRD difference between the 2 iliac spines.
Conclusions
We recommend that, for MRD measurement,
-an aspiration should be performed from each iliac spine, with each sample being quantified separately and an average obtained
-each measurement should involve at least 10 μg of good-quality DNA
The MRD value so obtained should have sufficient accuracy, sensitivity and precision for clinical decisions based upon the value to be made with confidence.
The described methods for MRD measurement should also be applicable to monitoring of all B-lymphocyte neoplasms, in addition to ALL.
Disclosures: Alexander Morley is a part-time employee of Morgen Research which holds a patent related to monoclonality testing.; Alexander Morley has provided unpaid consultation to Invivoscribe, a company which markets monoclonality testing kits.; Alexander Morley holds equity in Morgen Research. Drs Morley Brisco and Sykes hold equity in Monoquant PL, a company which is applying for a patent related to clonal repertoire analysis.; Morgen Research provides a grant to Flinders University for research in the area of minimal residual disease.; Drs Morley and Sykes are on the board of Monoquant PL. Dr Morley is managing director of Morgen Research.; Morgen Research receives royalties from Invivoscribe.
Author notes
Corresponding author


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal