Abstract
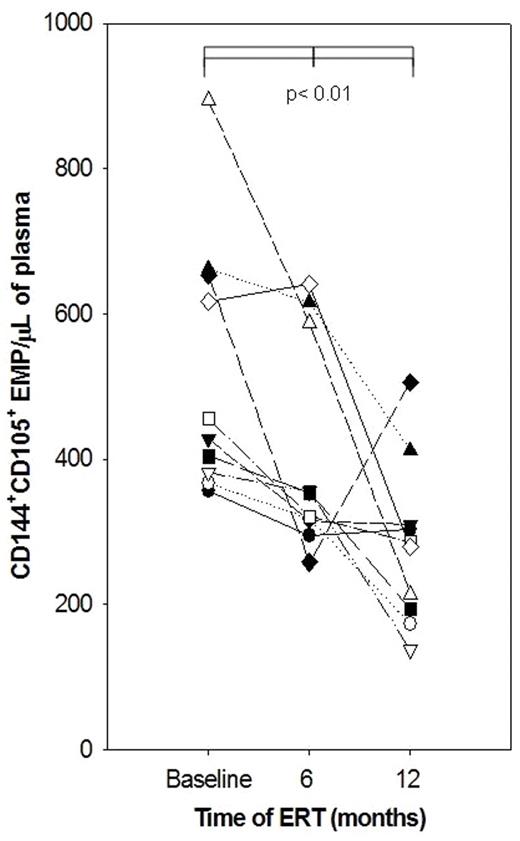
Fabry disease, an X-linked metabolic disorder, is caused by insufficient activity of the lysosomal enzyme alpha galactosidase A (α-gal A). This results in impaired catabolism of glycosphingolipids and their subsequent accumulation (Gb3) leading to endothelial dysfunction. Currently, enzyme replacement therapy (ERT) is the only available specific treatment for patients with Fabry disease. The identification of markers that could serve as indicators of disease severity, prognosis and possible effects of specific therapies is highly desirable. Elevated endothelial cell membrane microparticles in blood have been documented for various diseases with a vascular injury component. Thus, we investigated the presence of endothelial cell membrane derived microparticles (EMPs) in peripheral venous blood samples of 10 pediatric Fabry patients (9 males and 1 female) before they were started on ERT and at the end of 6 and 12 months into the treatment regimen of infusions every other week with agalsidase alfa (Shire Human Genetic Therapies, Cambridge, MA) at 0.2 mg/kg. Both the 6 and 12 month samples were collected 14 days after the last enzyme infusion, thus right before the next infusion. Median age of patients was 12 (range: 10 – 18); renal function, urinary protein excretion, and cardiac function and structure were normal. Carbamazepine or gabapentin were common medications. Patients were stable except for one who suffered recurrent symptomatic and lacunar ischemic strokes. Plasma from healthy age-matched volunteers (Ctrl, n=19) was used for comparison. For EMP analysis, which was done at the same time and blindly, we used a three-color flow cytometric assay (
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal