Abstract
Factor V has recently been observed to act as a synergistic anticoagulant cofactor in the activated Protein C (APC) pathway; however, the importance of factor V’s anticoagulant nature in vivo is poorly understood. A novel autosomal dominant bleeding disorder, which is characterized by excessive bleeding with trauma or surgery and menorrhagia in affected women, was identified in a large family (16 affected individuals) from east Texas. Affected members had a prolongation of their PT and/or aPTT. Clinical studies indicated normal activities and levels of all coagulation factors. Linkage analysis mapped the defective gene to 1q23–24 (LODmax 7.33), which contains the gene for coagulation factor V (FV). An alteration (A2440G)in the FV gene in exon 13, which encodes the entire B-domain of the wildtype protein, segregates with disease and was not present in 62 controls. Interestingly, this alteration falls on the 5′ splice site (TA/GT) of an alternative splicing variant confirmed by RT-PCR of control liver and leukocytes. Furthermore, immunoblot analysis demonstrated that this splice variant produces a low abundance, 250kD isoform of FV in control plasma. The A2440G alteration can theoretically form a higher efficiency consensus site for splicing (TG/GT). Semi-quantitative RT-PCR confirms this improved efficiency with an increase of splicing seen in patients’ RNA from leukocytes (n=3) versus controls (n=3). This translates into an approximately 20-fold increase in the 250kD isoform of FV seen in patients’ plasma (n=6) versus controls (n=5), while wildtype FV levels remain equivalent. Co-precipitation with Vitamin-K dependent coagulation factors from patient plasma suggests that this isoform interacts with known FV-interacting proteins (Figure 1). A recombinant of this splicing event (FV-703) exhibited normal activation by thrombin and normal prothrombinase activity when compared to the wildtype recombinant. Thrombin-activated FV-703 also showed normal degradation by APC, while intact FV-703 displayed an increased sensitivity to APC that was more striking in the presence of PS (Figure 2). We hypothesize that this novel isoform has a stronger affinity for APC in the presence of PS than wildtype FV and works primarily as a cofactor in the APC pathway to heighten the overall anticoagulant activity in the bloodstream. These data indicate that A2440G up-regulates an alternatively spliced transcript of FV, and increases a FV isoform that hinders coagulation as opposed to promoting it like its wildtype counterpart. As verified by this unique mutation, this novel alternative splicing of coagulation FV is an important physiological event that can result in disease if its delicate balance is disrupted.
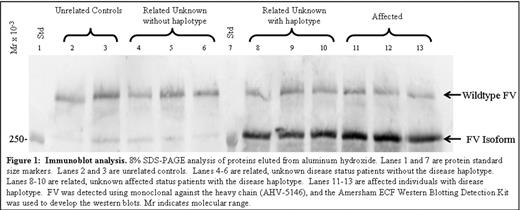
Immunoblot analysis. 8% SDS-PAGE analysis of proteins eluted from aluminum hydroxide Lanes 1 and 7 are protein standard size markers. Lanes 2 and 3 are unrelated controls. Lanes 4–6 are related, unknown disease status patients without the disease haplotype. Lanes 8–10 are related, unknown affected status patients with the disease haplotype. Lanes 11–13 are affected individuals with disease haplotype. FV was detected using monolconal against the heavy chain (AHV-5146), and the Arnerrham ECF Western Blotting Detection Kit was used to develop the western blots. Mr indicates molecular range.
Immunoblot analysis. 8% SDS-PAGE analysis of proteins eluted from aluminum hydroxide Lanes 1 and 7 are protein standard size markers. Lanes 2 and 3 are unrelated controls. Lanes 4–6 are related, unknown disease status patients without the disease haplotype. Lanes 8–10 are related, unknown affected status patients with the disease haplotype. Lanes 11–13 are affected individuals with disease haplotype. FV was detected using monolconal against the heavy chain (AHV-5146), and the Arnerrham ECF Western Blotting Detection Kit was used to develop the western blots. Mr indicates molecular range.
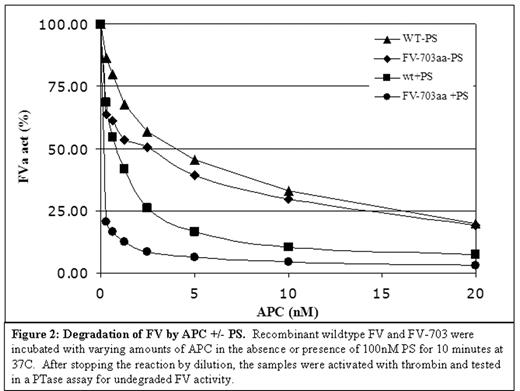
Degradation of FV by APC +/− PS. Recombinant wildtype FV and FV-703 were incubated with varying amounts of APC in the absence or presence of 100nM PS for 10 minutes at 37C. After stopping the reaction by dilution, the samples were activated with thrombin and tested in a PTase assay for undegraded FV activity.
Degradation of FV by APC +/− PS. Recombinant wildtype FV and FV-703 were incubated with varying amounts of APC in the absence or presence of 100nM PS for 10 minutes at 37C. After stopping the reaction by dilution, the samples were activated with thrombin and tested in a PTase assay for undegraded FV activity.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal