Abstract
During 1-year core phases of two deferasirox trials, doses were initially assigned by baseline liver iron concentration (LIC). In these regularly transfused patients (mean iron intake 0.37 mg/kg/day), a clear dose response was observed: deferasirox 20/30 mg/kg/day effectively maintained/reduced body iron, 10 mg/kg/day maintained body iron in patients with low transfusion rates, whereas 5 mg/kg/day removed less iron than added by ongoing transfusions. This post-hoc analysis evaluates the impact on serum ferritin (SF) of dose increases during the extension phases, in patients who initially received 5/10 mg/kg/day.
In patients who initially received deferasirox 5/10 mg/kg/day with increasing iron burden, doses were generally increased to 20–30 mg/kg/day during the first 6 months of the extension phase. Dose modifications for patients on 20/30 mg/kg/day were based on safety and efficacy parameters. SF was measured monthly.
480 patients (β-thalassemia, n=381; myelodysplastic syndromes, n=47; other anemias, n=52) initially received deferasirox 5/10 (n=119), 20 (n=136) or 30 (n=225) mg/kg/day in the core phase. Most continued on treatment in the extension phase, receiving deferasirox for a median of 2.6 years.
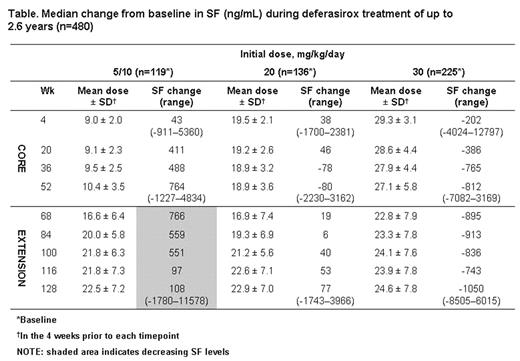
Median baseline SF values in the three dose cohorts were 1932, 2527 and 4158 ng/mL, respectively. During the core and extension phases, SF levels decreased in patients who received deferasirox 30 mg/kg/day and were maintained in patients who received 20 mg/kg/day. In contrast, SF steadily increased in patients who initially received 5/10 mg/kg/day. Subsequent dose escalation during the extension phase generally resulted in decreased SF levels (see shaded area of Table), returning close to baseline by data cut-off. A similar pattern was observed irrespective of age or underlying anemia.
Median change from baseline in S:F (ng/ml) during deferasirox treatment of up to 2.6 years (n=480)
A further 258 patients (deferoxamine arm in core phase) crossed over to deferasirox during the extension phase, receiving deferasirox for a median of 1.6 years. Comparable responses were observed in these patients versus those in the deferasirox cohort at corresponding doses.
Conclusions: SF steadily decreased following dose increases in regularly transfused patients who initially received deferasirox 5/10 mg/kg/day. This analysis confirms that 30 mg/kg/day effectively reduces body iron over the long term, whereas 20 mg/kg/day is effective in maintaining body iron levels in regularly transfused patients. Deferasirox dose can therefore be effectively tailored depending on the goal of therapy (maintenance or reduction in body iron levels).
Disclosures: H Maseruka, E Glimm and D Alberti are all employees of Novartis.; J Porter - Advisory Board for Exjade.; J Porter - conducting of clinical trials on Exjade; A Cohen - received research funding as an investigator in the trials described in the abstract as well as other studies sponsored by Novartis.; J Porter has received honoraria.; J Porter - Expert advisor to Novartis for registration of deferasirox in US (FDA) and Europe (EMEA).; J Porter - Speakers’ bureau for Exjade; MD Cappellini - Member of advisory board for Novartis (Exjade Trial 107); A Cohen - Member of the Study Monitoring Committee for two Novartis-sponsored Exjade clinical trials.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal