Abstract
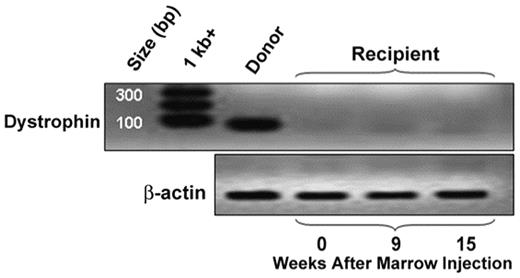
We sought to determine whether wild-type hematopoietic stem cells directly injected into muscle could restore dystrophin expression in a relevant pre-clinical canine model of Duchenne muscular dystrophy (DMD). Recipients that possessed the dystrophin mutation and thus exhibited the DMD phenotype were chosen. They were rendered tolerant to their dog leukocyte antigen (DLA)-matched unaffected littermates through myeloablative conditioning followed by hematopoietic stem cell transplantation (HCT). Donor bone marrow cells were collected (2.19e8 cells/kg) and injected directly under the aponeurosis of the superspinatus muscle of the DMD affected animals. Excisional muscle biopsies 5, 9 and 15 weeks following injection were examined with immunofluorescence and reverse-transcription-PCR for dystrophin protein and mRNA expression (figure), respectively, and failed to demonstrate increases over background. Consistent with these results, clonal satellite cell cultures from these biopsies at the initiation of myotube formation showed no detectable donor contributions. These findings lessen the enthusiasm for strategies relying on hematopoietic stem cell transdifferentiation to repair or regenerate skeletal muscle.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal