Abstract
Introduction: The Notch pathway regulates critical cell-fate decisions affecting the growth and development of human hematopoietic cells. Although Notch1 is a known T cell oncogene, we have discovered that Notch signaling behaves as a tumor suppressor in acute myeloblastic leukemia (AML) inducing growth arrest and apoptosis in both cell lines and patient samples. To characterize the mechanism of this effect we have evaluated the influence of the Notch pathway on key effectors of differentiation, cell cycle, and apoptosis in human AML.
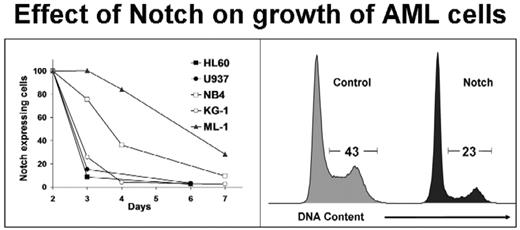
Results: Notch signaling induces rapid growth arrest and apoptosis in a panel of human AML cell lines representing a range of AML FAB subtypes (M2–M6). Specifically, activated Notch1 expression caused a 70–95% reduction in AML cells compared to controls (p<0.001) (Figure 1). Notch-mediated growth arrest occurred in 24–48 hours with cells accumulating in G0/G1. Apoptosis was demonstrated by a 3.8-fold increase in AnnexinV binding (p<0.004) and a 3-fold upregulation of caspase 3 activity (p=0.0002) within 24 hours. The caspase 3 activity was abolished by the caspase 8 inhibitor IETD (p<0.0001) suggesting a potential role for the extrinsic death pathway.
We also found that all four Notch receptors (1–4) are capable of inducing this effect, as is the Notch target gene HES1, suggesting a generalized Notch tumor suppressor effect in AML. Furthermore, Notch signaling through HES1 modulates the expression of key regulators of myeloid differentiation and cell cycle progression including downregulation of CEBPα 2.5-fold (p<0.02) and upregulation of p21WAF1 6-fold (p<0.004) suggesting potential mechanisms.
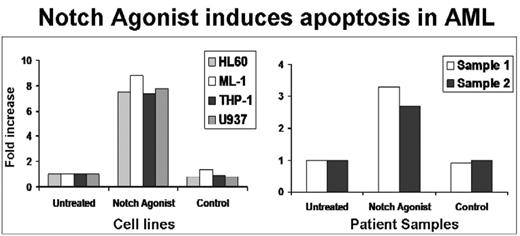
As a novel therapeutic approach, we synthesized Notch agonists which effectively induce Notch signaling with a >18-fold increase in HES1 expression (p<.0001). Exposure of human AML cell lines and primary patient AML samples to this Notch agonist for 24 hours led to a 3 to 9-fold increase in apoptosis (p<0.017) compared to controls (Figure 2).
Conclusions: We report here that Notch signaling is a novel tumor suppressor pathway in human AML. We demonstrate how Notch agonists can be used to induce growth arrest and apoptosis in human AML cell lines and patient AML samples. As a regulator of cell fate, proliferation and differentiation, Notch effectively disrupts multiple pathways in AML. We propose that Notch agonists represent a novel and feasible therapeutic approach in AML. Pre-clinical evaluation is underway.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal