Abstract
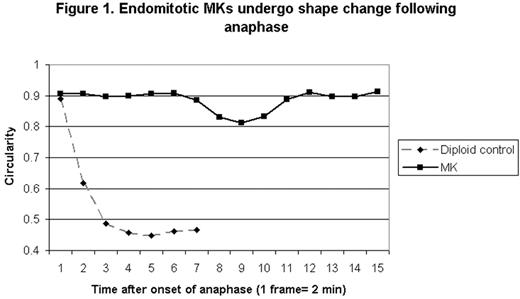
Megakaryocytes (MKs) develop high degrees of polyploidy in the course of normal maturation through a process called endomitosis. Previous studies have shown that endomitotic MKs abort anaphase after sister chromatid separation, and studies using fixed cells have shown that a midzone is formed in anaphase but that a well-developed cleavage furrow is not. However, studies in fixed cells may miss transient dynamic events that are critical for evaluating potential mechanisms of failed cytokinesis in endomitosis. Therefore we have used live cell imaging as a tool to further define the ability of primary endomitotic MKs to furrow during anaphase. We imaged mouse marrow MKs expressing tubulin fused to YFP, acquiring brightfield and fluorescent images every 2 minutes. Endomitotic cells were identified by the formation of a multipolar spindle, and anaphase was defined as the point at which separation of spindle poles was visible. The extent of cell shape change following anaphase was determined visually and also by measuring the circularity of the cell, with a perfectly round cell having a circularity value of 1. We found that 56% of endomitotic MKs underwent some shape change (n=25), visible as localized indentation, protrusion or elongation of the cell cortex, with an average reduction in circularity of 0.043 occurring a mean of 14.6 minutes after the initiation of anaphase. After an average of 7.8 minutes the cells generally reverted to a round shape without completing furrow ingression. In contrast, a typical mitotic control cell showed a reduction in circularity of 0.443 occurring 8 minutes after the start of anaphase and resulting in the formation of two cells (see Figure 1, representative cells). To determine if the shape change seen in endomitosis after anaphase is dependent on RhoA, we imaged MKs in the presence of the RhoA inhibitor C3 toxin. Although 2 μg/ml C3 toxin blocked cytokinesis in diploid control cells, it did not measurably alter the frequency (44%, n=16) or degree of shape change (0.039) in endomitotic MKs following anaphase, although treatment did appear to delay shape change (onset occurring a mean of 24.8 min after anaphase) and prolong the period of cortical contractility. Therefore although endomitotic MKs exhibit a phase of increased cortical contractility following anaphase, it is not solely dependent on the activity of RhoA and it does not result in sustained furrow ingression. Live cell imaging represents a powerful new tool for dissecting the mechanisms of endomitosis.
Endomitotic MKs undergo shape change following anaphase
Endomitotic MKs undergo shape change following anaphase
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal