Abstract
Background: GMA161 is a humanized version of a monoclonal anti-Fcγ-RIII antibody, 3G8, that was in clinical trial in the second half of the 1980s. Infusion of 25 mg of 3G8 (0.25–0.5mg/kg) resulted in transient but dramatic responses in approximately 50% of refractory ITP patients who did not respond to IVIG. It was unclear why certain patients did not respond. Since 3G8 is a mouse monoclonal antibody, it could not be reinfused because of the development of HAMA. Furthermore, there was marked neutropenia and depletion of NK cells (the CD16-expressing leukocytes) and there were significant fever-chill-vomiting reactions that were triggered by the antibody-Fcγ-RIII interaction. Preventing the reactions required a cocktail of methylprednisolone, diphenhydramine, acetaminophen and metaclopramide. GMA161, in addition to being humanized, has the Fc portion denuded of carbohydrates to reduce the binding of its Fc portion to Fc receptors.
Methods: The first cohort of 4 patients with chronic ITP (table) and platelet counts < 30,000/ul each received a single infusion of 0.1 mg/kg of GMA161 over 30 minutes.
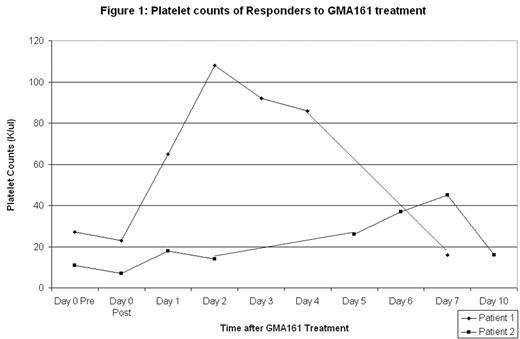
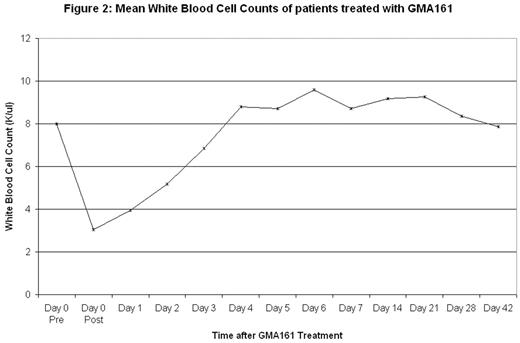
Results: Two of the 4 patients responded with peak platelet counts of 108,000/ul (from 27,000/ul) and 45,000/ul (from 11,000/ul) [figure 1]. Both had had ITP for > 20 years, failed splenectomy and also failed multiple previous therapies including rituximab, cyclophosphamide, steroids, danazol, and IVIg among others. The responses to GMA161 were short-lived, lasting between 7 and 10 days and, as shown in figure 1, the platelet counts peaked at slightly different times. Figure 2 illustrates the dramatic decrease in the WBC count occurring immediately after infusion but then rapidly returning to baseline; this decrease was apparent in all types of white cells, not just neutrophils. The first patient had marked chills, fever, and vomiting 2 hours after infusion; this was reminscent of reactions to 3G8. She received IV methylprednisolone with resolution. The second patient had mild-moderate nausea. The third and fourth patients received acetaminophen, diphenhydramine and ondansetron premedication and had no adverse events.
Conclusions: The findings are exciting because this cohort received the starting (lowest) dose of GMA161, 0.1mg/kg, and yet had reasonable activity. The toxicity was minimal with appropriate premedication. In the next cohort, better responses may be anticipated since they will be receiving 0.3 mg/kg. The hope would be that repeated dosing might have a more lasting effect, in at least a subset of patients.
GMA161: Cohort #1
| Patient ID . | Age (yrs) . | Sex . | Splenectomy . | Duration of Disease (ITP) Months . | Major Bleeding . | Major Diagnoses . | Previous Treatments . |
|---|---|---|---|---|---|---|---|
| #1 | 29 | Female | Yes | 90 | No | Anemia | 7 |
| #2 | 32 | Female | Yes | No | |||
| #3 | 70 | Male | Yes | 300 | No | Diabetes | 4 |
| #4 | 59 | Female | Yes | 120 | Yes (ICH) | Stroke/Asthma | 6 |
| Patient ID . | Age (yrs) . | Sex . | Splenectomy . | Duration of Disease (ITP) Months . | Major Bleeding . | Major Diagnoses . | Previous Treatments . |
|---|---|---|---|---|---|---|---|
| #1 | 29 | Female | Yes | 90 | No | Anemia | 7 |
| #2 | 32 | Female | Yes | No | |||
| #3 | 70 | Male | Yes | 300 | No | Diabetes | 4 |
| #4 | 59 | Female | Yes | 120 | Yes (ICH) | Stroke/Asthma | 6 |
Disclosures: GMA161 is not approved for use in ITP.; Krista Tibbs, Michael Watts, and Daniel Magilavy are employees of Genzyme.; Clinical research support provided to investigators.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal