The role of self-recognition in the maintenance of the peripheral CD4+ T-cell pool has been extensively studied, but no clear answer has so far emerged. Indeed, in studies of the role of self-major histocompatibility complex (MHC) molecules in CD4+ T-cell survival, several parameters must be taken into account when interpreting the results: (1) in a lymphopenic environment, observations are biased by concomitant proliferation of T cells arising in MHC-expressing mice; (2) the peripheral T-cell compartment is qualitatively and quantitatively different in nonlymphopenic, normal, and MHC class II-deficient mice; and (3) in C57BL/6 Aβ-/- mice (traditionally considered MHC class II-deficient), the Aα chain and the Eβ chain associate to form a hybrid AαEβ MHC class II molecule. In light of these considerations, we revisited the role of interactions with MHC class II molecules in the survival of peripheral CD4+ T cells. We found that the answer to the question “is self-recognition required for CD4+ T cells to survive?” is not a simple yes or no. Indeed, although long-term survival of CD4+ T cells does not depend on self-recognition in lymphopenic mice, interactions with MHC class II molecules are required for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. (Blood. 2006;108:270-277)
Introduction
The role of self-peptide/self-major histocompatibility complex (MHC) class II molecule complexes in the survival of peripheral CD4+ T cells has been extensively studied, but no clear answer has yet emerged.1-12 Adoptive transfer of mature T cells, thymus grafting, and more elegant experimental systems in which MHC molecules are transiently expressed in the thymus have been used to address this issue. Based on differential CD4+ T-cell recovery from lymphopenic mice with and without MHC class II expression, numerous groups have proposed that the survival of peripheral CD4+ T cells depends on permanent interactions with MHC class II molecules expressed on peripheral antigen-presenting cells.1-5,8,10 However, these conclusions are undermined by the concomitant expansion of T cells arising in MHC-expressing lymphopenic mice.13,14 Indeed, few of these reports clearly distinguished between the effects of MHC recognition on T-cell survival from lymphopenia-induced proliferation of T cells in response to self-peptide/self-MHC molecule complexes, a phenomenon previously described in neonatal mice15,16 and after transfer into lymphopenic hosts.17
More recently, several groups have observed that, in a nonlymphopenic environment, the kinetics of CD4+ T-cell disappearance is independent of the ability of recipient mice to express MHC class II molecules.6,7 They concluded that the survival of peripheral CD4+ T cells does not depend on T-cell antigen receptor (TCR) signaling induced by recognition of self-peptide/self-MHC molecule complexes. However, these studies are also open to criticism, because the similar pattern of CD4+ T-cell decline in the different mice could occur for different reasons such as competition and replacement in normal recipients, and death due to the absence of TCR contact with self-MHC molecules in mice lacking MHC class II molecules.18
Another criticism applying to most studies of CD4+ T-cell survival is that nearly all groups working in this field have considered C57BL/6 Aβ-/- mice to be MHC class II-deficient.3-7,11 In C57BL/6 mice, a point mutation in the I-Eα gene precludes the synthesis of the functional protein and the subsequent expression of the MHC class II molecule I-E. By disrupting the I-Aβ gene in these mice, one would expect to abrogate the expression of MHC class II molecules.19 However, we recently reported that, in C57BL/6 A β-/- mice, the Aα chain and the Eβ chain associate to form a hybrid AαEβ MHC class II molecule.20 This challenges the interpretation of all previous studies of CD4+ T-cell survival in which C57BL/6 Aβ-/- mice served as MHC class II molecule nonexpressing hosts.
Thus, in studies of the role of self-MHC molecules in CD4+ T-cell survival, one needs to take into account several parameters when interpreting the results: (1) in a lymphopenic environment, peripheral T-cell proliferation and survival may rely on different mechanisms; (2) the peripheral T-cell compartment is qualitatively and quantitatively different in nonlymphopenic, normal, and MHC class II-deficient mice; and (3) in C57BL/6 Aβ-/- mice, the Aα chain and the Eβ chain associate to form a hybrid AαEβ MHC class II molecule.
In light of these considerations, we decided: (1) to label purified CD4+ T cells with CFSE before transfer, in order to discriminate between survival and proliferation; (2) to conduct sequential transfer experiments in order to assess the role of defined T-cell competitors in CD4+ T-cell survival; and (3) to use mice in which neither conventional nor hybrid MHC class II molecules are expressed (MHC IIΔ/Δ mice, which lack both the α and β chains of I-A and I-E MHC class II molecules21 ).
In the present paper, we thus revisited the role of interactions with MHC class II molecules in the survival of peripheral CD4+ T cells. We found that the answer to the question “is selfrecognition required for CD4+ T cells to survive?” is not a simple yes or no. Indeed, the survival and proliferative capacities of transferred polyclonal CD4+ T cells differ strongly according to the ability of recipient mice to produce endogenous T cells (lymphopenic versus T-cell-containing mice) and to express MHC class II molecules.
Materials and methods
Mice
C57BL/6 mice were obtained from Centre d'élevage Janvier (Le Genest Saint Isle, France), and C57BL/6 CD3ϵ-deficient mice (CD3ϵ-/- mice), C57BL/6 CD45.1 mice, and C57BL/6 β -/-2m mice were from Centre de Développement des Techniques Avancées pour l'Expérimentation Animale (Orléans, France). MHC IIΔ/Δ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained in our animal facilities. C57BL/6 CD3ϵ-deficient mice were crossed with MHC IIΔ/Δ mice to generate C57BL/6 CD3ϵ/MHC II double-deficient mice (CD3ϵ-/-IIΔ/Δ mice). CD3ϵ-/-IIΔ/Δ mice were then crossed with β2m-deficient mice to generate C57BL/6 CD3ϵ/β2m/MHC II triple-deficient mice (CD3ϵ-/-β -/-2m IIΔ/Δ mice).
Adoptive transfer of T cells
Lymph node cells were depleted of macrophages, granulocytes, and CD8+ and CD4+ T cells by incubating them first with anti-CD11b (Mac-1) antibody (Ab), anti-GR1 (8C5) Ab, and anti-CD8 (53-6.7) or CD4 (GK1.5) Ab, and then with magnetic beads coated with antirat Ab (Dynal, Great Neck, NY). B cells were removed by using magnetic beads coated with anti-mouse immunoglobulin (Ig) Ab (Dynal). In some experiments (Figure 3), purified CD4+ T cells from C57BL/6 mice were labeled with biotinylated anti-CD25 (clone PC61) and PE anti-CD44 (clone 1M7). CD4+ CD25-CD44- naive T cells were then purified by sorting in a FACSVantage flow cytometer (BD Biosciences, Mountain View, CA). Purified CD4+ T cells were injected intravenously into recipient mice. When indicated, CD4+ T cells were labeled with CFSE (Molecular Probes, Eugene, OR) before injection. In some other experiments (Figure 6), purified CD8+ or CD4+ T cells (50 × 106) were injected 3 days before the CFSE-labeled CD4+ T-cell cohort. In the experiment represented in Figure 7C, 7-week-old C57BL/6 mice were thymectomized or sham-thymectomized 35 days before CD4+ T-cell transfer.
Cell-surface staining and flow cytometry
Spleen and lymph nodes were recovered, pooled for cell preparation, and analyzed at various times after CD4+ T-cell transfer. Lymph nodes and spleen were homogenized with a nylon cell strainer (Falcon, Franklin Lakes, NJ) in phosphate-buffered saline (PBS) + 5% fetal calf serum (FCS) + 0.2% NaN3, and then distributed in 96-well U-bottom microplates (6 × 106 cells per well). Staining was performed in ice for 30 minutes per step. Antibodies were purchased from BD Pharmingen (San Diego, CA) unless otherwise indicated. The following antibody combinations were used to characterize transferred CFSE-labeled lymph node CD4+ T cells: peridinin chlorophyll protein (PerCP) anti-CD4, and biotinylated anti-TCRβ, anti-CD44, anti-CD45.1, with allophycocyanin-streptavidin development (BD Pharmingen).
Four-color immunofluorescence was analyzed by using a FACSCalibur cytometer (Becton Dickinson, San Jose, CA). List-mode data files were analyzed by using Cellquest software (Becton Dickinson).
Analysis of ζ tyrosine phosphorylation
Purified CD4+ T cells were washed twice with ice-cold PBS and placed in lysis buffer containing 1% NP-40, 10 mM Tris-HCl (pH 7.2), 140 mM NaCl, 2 mM EDTA, 5 mM iodoacetamide, 1 mM Na3 VO4, and protease inhibitors for 20 minutes on ice. Nuclear debris was removed and the resulting supernatants were collected. Protein was then eluted in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and analyzed by immunoblotting. Two immunoblots were prepared with each supernatant; the first was developed with 4G10 Ab (a mouse Ab to phosphotyrosine; Upstate Biotechnology, Lake Placid, NY), and the second with 6B-10.2 Ab (a mouse Ab to ζ chains; Santa Cruz Biotechnology, Santa Cruz, CA). Both immunoblots were then developed with peroxidase-linked goat anti-mouse IgG and analyzed by chemiluminescence. Quantitative data were obtained from films exposured with a Gel Doc system (Biorad, Hercules, CA).
In vivo CD8+ T-cell depletion
Six-week-old C57BL/6 mice were injected intraperitoneally with 50 μg of purified anti-CD8 Ab (53-6.7) every 2 days for 4 weeks. We verified that such a treatment selectively depletes peripheral CD8+ T cells, without affecting thymic differentiation (data not shown).
Results
Survival of CD4+ T cells in a lymphopenic environment does not depend on interactions with MHC class II molecules
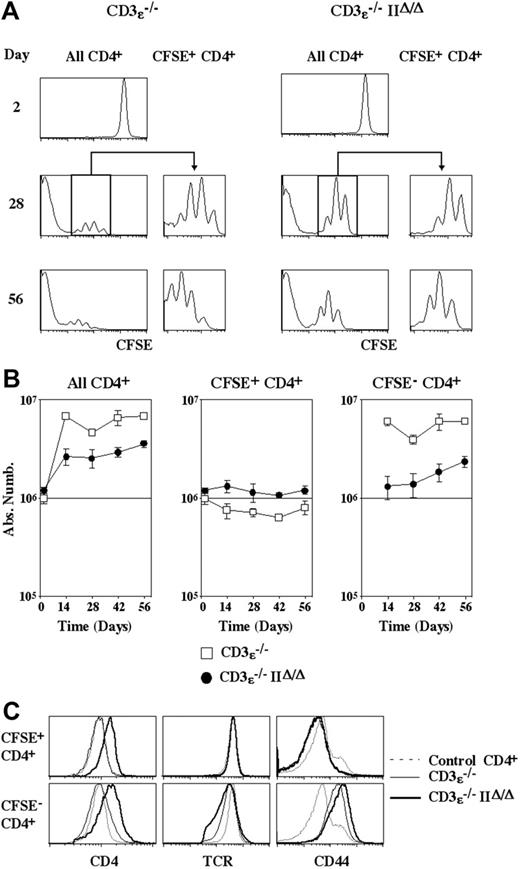
In order to study the fate of CD4+ T cells transferred into lymphopenic mice as a function of the expression or nonexpression of MHC class II molecules, we injected CFSE-labeled purified CD4+ T cells into CD3ϵ-/- mice and CD3ϵ-/-IIΔ/Δ mice. No cell division was detected for the first 2 days after transfer (Figure 1A). Four weeks later, in both recipients, some recovered cells were found to have undergone a limited number of divisions, whereas the intracytoplasmic dye had totally disappeared from others. Thus, as previously described by us and others,20,22-24 we confirmed that CD4+ T cells from normal C57BL/6 mice can be subdivided in 2 subsets with respect to their behavior after transfer into lymphopenic mice expressing MHC class II molecules. The first subset (CFSE+ CD4+ T cells), corresponding to the vast majority of transferred CD4+ T cells, cycles very slowly. The second subset (CFSE- CD4+ T cells) is generated by strong expansion of a small proportion of injected CD4+ T cells. Surprisingly, both subsets were also identified after CD4+ T-cell transfer into CD3ϵ-/-IIΔ/Δ mice.
Absolute numbers of recovered CD4+ T cells were then calculated as a function of their CFSE labeling status (Figure 1B). In both recipients the absolute numbers of recovered CD4+ T cells increased with time and, as stated, this reflected the strong proliferation of a minority of injected cells (giving rise to CFSE- CD4+ T cells). More precisely, in CD3ϵ-/- mice, the absolute number of CFSE- CD4+ T cells had already reached a plateau 2 weeks after transfer, whereas it still gradually increased with time in CD3ϵ-/-IIΔ/Δ mice. Thus, the kinetics of CFSE- CD4+ T-cell generation differed according to the ability of recipient mice to express MHC class II molecules. By contrast, the behavior of the bulk of transferred CD4+ T cells (CFSE+ CD4+ T cells) was similar in the 2 recipients. Indeed, CFSE+ CD4+ T cells underwent a limited number of divisions and their number remained fairly constant. Thus, CD4+ T-cell survival does not seem to depend on MHC class II interactions in a lymphopenic environment.
Survival of CD4+T cells in a lymphopenic environment does not depend on interactions with MHC class II molecules. CFSE-labeled lymph node CD4+ T cells (5 × 106) from normal C57BL6 mice were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. At various times after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. (A) CFSE fluorescence histograms of CD4+ TCRhicells are shown as a function of time after transfer. Histograms on the right are gated on CFSE+ CD4+ TCRhicells. (B) Absolute numbers of total, CFSE+, and CFSE- CD4+ TCRhicells are shown as a function of time after transfer. Data represent mean ± SD. (C) CD4, TCR, and CD44 fluorescence histograms of CFSE+ and CFSE- CD4+ T cells recovered 28 days after transfer are shown in comparison with CD4, TCR, and CD44 fluorescence histograms of control CD4+ T cells from normal C57BL/6 mice.
Survival of CD4+T cells in a lymphopenic environment does not depend on interactions with MHC class II molecules. CFSE-labeled lymph node CD4+ T cells (5 × 106) from normal C57BL6 mice were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. At various times after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. (A) CFSE fluorescence histograms of CD4+ TCRhicells are shown as a function of time after transfer. Histograms on the right are gated on CFSE+ CD4+ TCRhicells. (B) Absolute numbers of total, CFSE+, and CFSE- CD4+ TCRhicells are shown as a function of time after transfer. Data represent mean ± SD. (C) CD4, TCR, and CD44 fluorescence histograms of CFSE+ and CFSE- CD4+ T cells recovered 28 days after transfer are shown in comparison with CD4, TCR, and CD44 fluorescence histograms of control CD4+ T cells from normal C57BL/6 mice.
The phenotype of CD4+ T cells recovered 28 days after transfer was then analyzed (Figure 1C). In both CD3ϵ-/- mice and CD3ϵ-/- IIΔ/Δ mice, CD44 up-regulation and TCR down-regulation were restricted to CFSE- CD4+ T cells. Thus, only the progeny of the few CD4+ T cells that strongly proliferate exhibit an effector-like phenotype. In contrast, the bulk of injected cells cycles slowly, survives, and retains a naive phenotype. Interestingly, in mice lacking MHC class II molecules, CD4 expression was up-regulated in both CFSE+ and CFSE- cells.
The kinetics of CFSE- CD4+ T-cell generation was different when CD4+ T cells were transferred into CD3ϵ-/- mice and CD3ϵ-/-IIΔ/Δ mice. This suggested that the frequency of CD4+ T cells that are able to proliferate strongly in a lymphopenic environment might be influenced by MHC class II molecule expression. To examine this possibility, different amounts of CD4+ T cells were injected into CD3ϵ-/- mice and CD3ϵ-/-IIΔ/Δ mice. Twenty-eight days after transfer, CFSE+ CD4+ T-cell recovery was identical in both recipients, and was strictly proportional to the absolute number of injected CD4+ T cells, confirming that CD4+ T-cell survival in a lymphopenic environment does not rely on interactions with MHC class II molecules. By contrast, the absolute number of recovered CFSE- CD4+ T cells depended on the expression of MHC class II molecules (Figure 2B). In CD3ϵ-/- mice, injection of 105 CD4+ T cells was sufficient for maximal CFSE- CD4+ T-cell recovery 28 days later, whereas transfer of 5 × 106 CD4+ T cells was not enough to reach this plateau in CD3ϵ-/-IIΔ/Δ mice. 5 hundred times more CD4+ T cells had to be injected into CD3ϵ-/-IIΔ/Δ recipient mice in order to recover the same absolute number of CFSE- CD4+ T cells as in CD3ϵ-/- mice. Together these findings indicate that the frequency of CD4+ T cells that are able to strongly proliferate in a lymphopenic environment is strongly diminished (500-fold) in the absence of MHC class II molecule expression.
Generation of CFSE- CD4+ T cells in the absence of MHC class II expression is not related to preferential expansion of memory CD4+ T cells
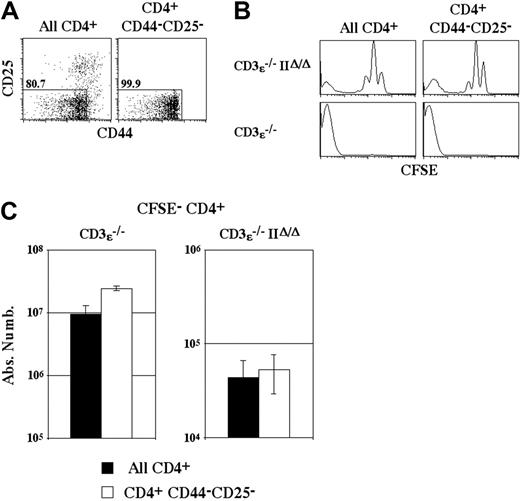
In the absence of MHC class II molecule expression, the frequency of CD4+ T cells that are able to proliferate strongly in a lymphopenic environment is greatly reduced (Figure 2B). This lower frequency could be explained by the possibility that a proportion of both naive and memory CD4+ T cells undergo strong proliferation after transfer into MHC class II molecule-expressing mice, whereas only memory CD4+ T cells would expand strongly in CD3ϵ-/IIΔ/Δ recipient mice.
The frequency of CD4+ T cells giving rise to CFSE- CD4+ T cells is strongly diminished in the absence of MHC class II molecule expression. Various amounts (103to 5 × 106) of CFSE-labeled lymph node CD4+ T cells from normal C57BL6 mice were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/- MHC IIΔ/Δ mice. Twenty-eight days after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. (A) Absolute numbers of recovered CFSE+ CD4+ TCRhicells are shown as a function of the number of injected cells. (B) Absolute numbers of recovered CFSE- CD4+ TCRhi cells are shown as a function of the number of injected cells.
The frequency of CD4+ T cells giving rise to CFSE- CD4+ T cells is strongly diminished in the absence of MHC class II molecule expression. Various amounts (103to 5 × 106) of CFSE-labeled lymph node CD4+ T cells from normal C57BL6 mice were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/- MHC IIΔ/Δ mice. Twenty-eight days after transfer, lymph nodes and spleen were recovered and pooled, and single-cell suspensions were prepared. (A) Absolute numbers of recovered CFSE+ CD4+ TCRhicells are shown as a function of the number of injected cells. (B) Absolute numbers of recovered CFSE- CD4+ TCRhi cells are shown as a function of the number of injected cells.
To test this hypothesis, total CD4+ T cells and purified naive CD4+ T cells (CD44-CD25-CD4+ T cells) were injected into CD3ϵ-/- mice and CD3ϵ-/-IIΔ/Δ mice (Figure 3A). The proliferation of injected cells was studied 14 days after transfer, before the occurrence of the wasting disease induced by transfer of naive CD4+ T cells alone.25 Independently of the ability of recipient mice to express MHC class II molecules, the transfer of both total and naive CD4+ T cells led to the generation of a subset of CFSE- CD4+ T cells (Figure 3B). No difference in the absolute numbers of recovered CFSE- CD4+ T cells was observed in CD3ϵ-/-IIΔ/Δ recipient mice whether total or naive CD4+ T cells were injected (Figure 3C). Thus, CFSE- CD4+ T-cell generation in CD3ϵ-/-IIΔ/Δ mice is not related to preferential expansion of memory CD4+ T cells.
CFSE- CD4+ T-cell generation depends strictly on interactions with MHC molecules
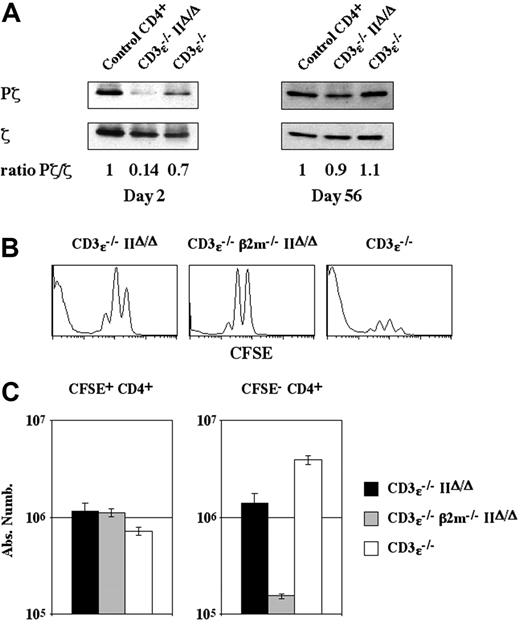
Some naive CD4+ T cells were able to proliferate strongly and to convert to a memory-like phenotype in a lymphopenic environment, even in the absence of MHC class II molecule expression. We thus investigated whether T-cell receptor signaling was required for CFSE- CD4+ T-cell generation by measuring the phosphorylation status of TCRζ chains on CD4+ T cells injected into CD3ϵ-/- mice and CD3ϵ-/-IIΔ/Δ mice.
Two days after transfer into CD3ϵ-/-IIΔ/Δ mice, the level of ζ phosphorylation was strongly diminished in CD4+ T cells (Pζ/ζ ratio, 0.14; Figure 4A). At this time, recovered CD4+ T cells had not yet divided and thus corresponded to the bulk of injected CD4+ T cells that survive and retain a naive phenotype (CFSE+ CD4+ T cells; Figure 1). Thus, in a lymphopenic environment, the survival of naive CD4+ T cells was not dependent on TCR-mediated signals.
CFSE- CD4+ T-cell generation in the absence of MHC class II molecule expression is not related to preferential expansion of memory CD4+ T cells. (A) Lymph node CD4+ T cells from C57BL/6 mice were purified and naive CD4+ T cells were electronically sorted on the basis of their nonexpression of CD25 and their low or absent expression of CD44. (B) CFSE-labeled CD4+ T cells (106) or 106purified naive CFSE-labeled CD4+ T cells (CD4+ CD44-CD25-) were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. CFSE fluorescence histograms of CD4+ TCRhicells recovered 14 days after transfer are shown. (C) Fourteen days after transfer the absolute numbers of CFSE- CD4+ TCRhicells were calculated. Data represent mean ± SD
CFSE- CD4+ T-cell generation in the absence of MHC class II molecule expression is not related to preferential expansion of memory CD4+ T cells. (A) Lymph node CD4+ T cells from C57BL/6 mice were purified and naive CD4+ T cells were electronically sorted on the basis of their nonexpression of CD25 and their low or absent expression of CD44. (B) CFSE-labeled CD4+ T cells (106) or 106purified naive CFSE-labeled CD4+ T cells (CD4+ CD44-CD25-) were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. CFSE fluorescence histograms of CD4+ TCRhicells recovered 14 days after transfer are shown. (C) Fourteen days after transfer the absolute numbers of CFSE- CD4+ TCRhicells were calculated. Data represent mean ± SD
CFSE- CD4+ T-cell generation depends strictly on interactions with MHC molecules. (A) Lymph node CD4+ T cells (5 × 106) were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. Two or 56 days after transfer, ζ protein levels (ζ) and the extent of ζ chain phosphorylation (Pζ) in recovered CD4+ T cells were determined by immunoblotting, and the Pζ/ζ ratio was calculated. Purified CD4+ T cells from C57BL/6 mice were used as controls. (B-C) CFSE-labeled lymph node CD4+ T cells (5 × 106) from C57BL/6 mice were injected intravenously into CD3ϵ-/- mice and CD3ϵ-/-MHC IIΔ/Δ mice. CFSE-labeled lymph node CD4+β -/- T cells (5 × 106) from C57BL/6 β2m-/- mice were injected intravenously into CD3ϵ-/- β2m-/- MHC IIΔ/Δ mice. CFSE fluorescence histograms of recovered CD4+ TCRhicells are shown 28 days after transfer (B). Twenty-eight days after transfer the absolute numbers of CFSE+ and CFSE- CD4+ TCRhi cells were calculated (C); data represent mean ± SD.
CFSE- CD4+ T-cell generation depends strictly on interactions with MHC molecules. (A) Lymph node CD4+ T cells (5 × 106) were injected intravenously into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. Two or 56 days after transfer, ζ protein levels (ζ) and the extent of ζ chain phosphorylation (Pζ) in recovered CD4+ T cells were determined by immunoblotting, and the Pζ/ζ ratio was calculated. Purified CD4+ T cells from C57BL/6 mice were used as controls. (B-C) CFSE-labeled lymph node CD4+ T cells (5 × 106) from C57BL/6 mice were injected intravenously into CD3ϵ-/- mice and CD3ϵ-/-MHC IIΔ/Δ mice. CFSE-labeled lymph node CD4+β -/- T cells (5 × 106) from C57BL/6 β2m-/- mice were injected intravenously into CD3ϵ-/- β2m-/- MHC IIΔ/Δ mice. CFSE fluorescence histograms of recovered CD4+ TCRhicells are shown 28 days after transfer (B). Twenty-eight days after transfer the absolute numbers of CFSE+ and CFSE- CD4+ TCRhi cells were calculated (C); data represent mean ± SD.
Two months after transfer, the level of ζ phosphorylation in CD4+ T cells recovered from CD3ϵ-/-IIΔ/Δ mice had strongly increased, approaching the level found in CD4+ T cells freshly isolated from normal C57BL/6 mice. The high level of ζ phosphorylation would reflect the phosphorylation status of this protein in the major subset of CD4+ T cells recovered at these late time-points (ie, CFSE- CD4+ T cells). These results point to the existence of an MHC molecule in CD3ϵ-/-IIΔ/Δ mice, with which some transferred CD4+ T cells need to interact in order to expand strongly.
Given the lack of MHC class II molecules in CD3ϵ-/-IIΔ/Δ mice, we examined whether MHC class I molecules might influence the behavior of CD4+ T cells transferred into CD3ϵ-/-IIΔ/Δ mice. We therefore transferred CD4+ T cells into CD3ϵ-/-mice, CD3ϵ-/-IIΔ/Δ mice, and CD3ϵ-/-β2m-/-IIΔ/Δ mice. Twenty-eight days after transfer, we found that the lack of MHC class I molecule expression did not affect the weak proliferation (Figure 4B) or survival of the bulk of transferred cells (CFSE+ CD4+ T cells) (Figure 4C), whereas the behavior of the few CD4+ T cells that strongly proliferated and gave rise to CFSE- CD4+ cells was markedly affected. Indeed, in CD3ϵ-/-β2m-/-IIΔ/Δ recipient mice the absolute number of recovered CFSE- CD4+ T cells was 10-fold lower than in CD3ϵ-/-IIΔ/Δ recipient mice. Thus, CFSE- CD4+ T-cell generation depends on interactions with MHC class II molecules in CD3ϵ-/- mice and, more surprisingly, on interactions with MHC class I molecules in CD3ϵ-/-IIΔ/Δ mice. Leaky expression of MHC class I molecules in the absence of β2m expression may account for the few remaining CFSE- CD4+ T cells detected 28 days after transfer in CD3ϵ-/-β2m-/-IIΔ/Δ mice. Taken together, these results suggest that interactions with MHC molecules are strictly required for CFSE- CD4+ T-cell generation.
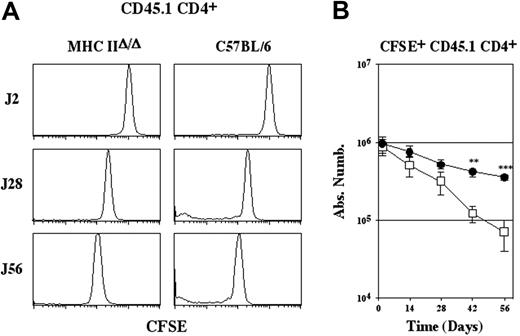
Survival of CD4+ T cells depends on interactions with MHC class II molecules in a nonlymphopenic environment. CFSE-labeled lymph node CD4+ T cells (5 × 106) from C57BL/6 CD45.1 mice were injected intravenously into MHC IIΔ/Δ mice and C57BL/6 CD45.2 mice. (A) CFSE fluorescence histograms of CD45.1 CD4+ TCRhi cells are shown as a function of time after transfer. (B) Absolute numbers of recovered CFSE+ CD45.1 CD4+ TCRhicells are shown as a function of time after transfer. Data represent mean ± SD. Statistically significant differences were assessed using Student t test. **P < .01; ***P < .001.• indicates C57BL/6; □, MHC IIΔ/Δ.
Survival of CD4+ T cells depends on interactions with MHC class II molecules in a nonlymphopenic environment. CFSE-labeled lymph node CD4+ T cells (5 × 106) from C57BL/6 CD45.1 mice were injected intravenously into MHC IIΔ/Δ mice and C57BL/6 CD45.2 mice. (A) CFSE fluorescence histograms of CD45.1 CD4+ TCRhi cells are shown as a function of time after transfer. (B) Absolute numbers of recovered CFSE+ CD45.1 CD4+ TCRhicells are shown as a function of time after transfer. Data represent mean ± SD. Statistically significant differences were assessed using Student t test. **P < .01; ***P < .001.• indicates C57BL/6; □, MHC IIΔ/Δ.
Survival of CD4+ T cells depends on interactions with MHC class II molecules in a nonlymphopenic environment
Most CD4+ T cells that survive in a lymphopenic environment do so independently of interactions with MHC class II molecules (Figures 1-2). However, in lymphopenic conditions, donor CD4+ T cells do not have to compete with a full preformed pool of endogenous T cells, or with continuous thymic output. For these reasons, we chose to use endogenous T-cell-containing recipients to continue our study of the role of MHC class II molecules in the survival of peripheral CD4+ T cells.
CD4+ T cells from C57BL/6 CD45.1 mice were injected into MHC IIΔ/Δ mice and C57BL/6 mice (Figure 5). The slow proliferation of the bulk of transferred CD4+ T cells (CFSE+ CD4+ T cells) was completely inhibited in both recipient mice. CFSE- CD4+ T-cell generation was not detected in MHC IIΔ/Δ mice and markedly reduced in control C57BL/6 mice (Figure 5A). Absolute numbers of recovered CFSE+ CD45.1 CD4+ T cells were then calculated (Figure 5B). Although transferred CD4+ T cells eventually disappeared in both recipient mice, the half-life of CD4+ T cells transferred into C57BL/6 mice (44.7 days) was twice as long as the half-life of CD4+ T cells transferred into MHC IIΔ/Δ mice (24.1 days) (Figure 5B). Interestingly, the half-life of CD4+ T cells transferred into mice lacking the expression of MHC class II molecules is quite similar to the half-life of CD4+ T lymphocytes stripped of their TCRs.8,9 Thus, interactions with MHC class II molecules allow CD4+ T cells to persist for longer in nonlymphopenic mice. These results suggest that MHC class II molecules play a role in peripheral CD4+ T-cell survival in physiologic conditions.
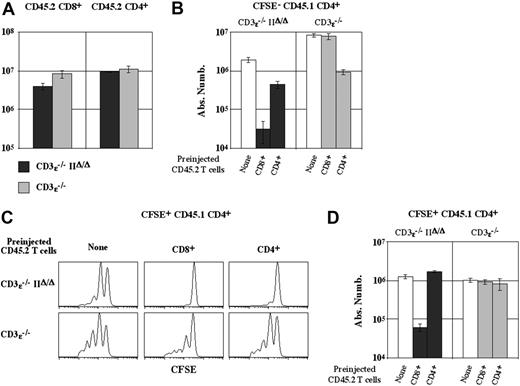
CD8+T lymphocytes determine the threshold of the MHC class II molecule requirement for CD4+T-cell survival. CFSE-labeled lymph node CD4+ T cells (5 × 106) from C57BL/6 CD45.1 mice were transferred into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. In some cases, host mice were injected with 50 × 106lymph node CD45.2 CD8+ or CD4+ T cells 3 days before CD45.1 CD4+ T-cell transfer. (A) Absolute numbers of recovered CD45.2 CD8+ and CD4+ T cells are shown 28 days after CD45.1 CD4+ T-cell transfer. (B) Absolute numbers of CFSE- CD45.1 CD4+ TCRhicells are shown 28 days after their transfer as a function of the CD4/8 phenotype of preinjected CD45.2 T cells. (C) CFSE fluorescence histograms of CFSE+ CD45.1 CD4+ TCRhicells are shown 28 days after their transfer as a function of the CD4/8 phenotype of preinjected CD45.2 T cells. (D) Absolute numbers of CFSE+ CD45.1 CD4+ TCRhicells are shown 28 days after their transfer as a function of the CD4/8 phenotype of preinjected CD45.2 T cells. (A-B, D) Data represent mean ± SD.
CD8+T lymphocytes determine the threshold of the MHC class II molecule requirement for CD4+T-cell survival. CFSE-labeled lymph node CD4+ T cells (5 × 106) from C57BL/6 CD45.1 mice were transferred into CD3ϵ-/-mice and CD3ϵ-/-MHC IIΔ/Δ mice. In some cases, host mice were injected with 50 × 106lymph node CD45.2 CD8+ or CD4+ T cells 3 days before CD45.1 CD4+ T-cell transfer. (A) Absolute numbers of recovered CD45.2 CD8+ and CD4+ T cells are shown 28 days after CD45.1 CD4+ T-cell transfer. (B) Absolute numbers of CFSE- CD45.1 CD4+ TCRhicells are shown 28 days after their transfer as a function of the CD4/8 phenotype of preinjected CD45.2 T cells. (C) CFSE fluorescence histograms of CFSE+ CD45.1 CD4+ TCRhicells are shown 28 days after their transfer as a function of the CD4/8 phenotype of preinjected CD45.2 T cells. (D) Absolute numbers of CFSE+ CD45.1 CD4+ TCRhicells are shown 28 days after their transfer as a function of the CD4/8 phenotype of preinjected CD45.2 T cells. (A-B, D) Data represent mean ± SD.
CD8+ T lymphocytes restrict the size of the peripheral CD4+ T-cell compartment
Normal mice contain both CD4+ and CD8+ T lymphocytes, whereas the peripheral T-cell compartment of MHC IIΔ/Δ mice is mainly composed of CD8+ T cells. As the nature and quantity of potential endogenous competitors differ between normal mice and MHC IIΔ/Δ mice, any comparison of the behavior of CD4+ T cells transferred into these 2 recipients might fail to identify the true role of MHC class II molecules in peripheral CD4+ T-cell survival. Therefore, we then investigated the survival capacity of CD4+ T cells as a function of MHC class II molecule expression and of the nature of “endogenous” T-cell competitors.
CD4+ T cells from C57BL/6 CD45.1 mice were transferred into CD3ϵ-/-IIΔ/Δ mice and CD3ϵ-/- mice, both of which had already been transferred with CD45.2 CD8+ or CD4+ T cells. The proliferation and survival of CD45.1 CD4+ T cells were assessed 28 days after their transfer. We first verified that CD45.2 CD8+ and CD45.2 CD4+ T-cell recoveries were not significantly different between the 2 recipient mice (Figure 6A).
We then examined whether the expansion of the minor CD4+ T-cell subset that generates CFSE- CD4+ T cells was modified in these conditions. As stated (Figures 3-4), CFSE- CD4+ T-cell generation depends strictly on interactions with MHC molecules (mainly MHC class II molecules in MHC class II-expressing mice, and MHC class I molecules in mice lacking MHC class II molecules). Accordingly, the proliferation and subsequent expansion of the minor CD4+ T-cell subset that produces CFSE- CD4+ T cells were both affected by the presence of preinjected CD4+ T cells in CD3ϵ-/- mice and by the presence of preinjected CD8+ T cells in CD3ϵ-/-IIΔ/Δ mice (Figure 6B).
Finally, we studied the fate of the bulk of the second cohort of injected CD4+ T cells (CFSE+ CD45.1 CD4+). In CD3ϵ-/-IIΔ/Δ-recipient mice, both T-cell competitors (CD45.2 CD4+ and CD8+ T cells) completely inhibited the slow proliferation of CFSE+ CD4+ T cells (Figure 6C). However, only pretransferred CD8+ T cells affected their survival (Figure 6D). Thus, surprisingly, CD4+ T cells compete less efficiently than CD8+ T cells for the non-MHC factor that permits their survival in lymphopenic mice. In MHC class II molecule-expressing mice (CD3ϵ-/ recipient mice), CD4+ T-cell competitors partially inhibited the slow proliferation of CFSE+ CD4+ T cells (Figure 6C) but did not interfere with their ability to survive (Figure 6D). Surprisingly, even when we preinjected sufficient CD4+ T cells to reach the absolute number of CD4+ T cells recovered from the peripheral compartment of a normal C57BL/6 mouse (ie, 25 × 106 CD4+ T cells), the behavior of the second cohort of CD4+ T cells was unaffected, and the numbers of all CD4+ T cells (the first and second cohorts) remained stable throughout the study period (data not shown). Similarly, in the presence of MHC class II molecules, preinjected CD8+ T cells did not interfere with the survival of CD4+ T cells.
Maintenance of the peripheral CD4+ T-cell pool seems to rely on different mechanisms in the presence and absence of CD8+ T cells. In the absence of CD8+ T cells, self-recognition is not required for the maintenance of CD4+ T-cell numbers. In contrast, interactions with MHC molecules become necessary to ensure the survival of CD4+ T cells in CD8+ T-cell-containing mice. The most logical explanation is that, in CD8+ T-cell-containing mice that lack MHC class II molecules, injected CD4+ T cells have to compete with preinjected CD8+ T cells for the soluble factor that permits their survival in a lymphopenic environment. We thus hypothesized that in MHC class II-expressing mice that do not contain CD8+ T cells, the 2 signals would synergize to maintain a larger CD4+ T-cell pool than in normal mice (CD8+ T-cell-containing MHC class II-expressing mice). To test this possibility, we estimated the size of the peripheral CD4+ T-cell compartment both in C57BL/6 mice lacking MHC class I molecules and therefore devoid of CD8+ T lymphocytes (C57BL/6 β-/-2m mice) and in normal C57BL/6 mice treated with anti-CD8 antibodies (Figure 7). In both experimental models we found a significant augmentation of the CD4+ T-cell pool compared with control C57BL/6 mice (Figure 7A-B). Interestingly, in both models the CD4+ T-cell compartment was augmented but not qualitatively modified (as assessed by CD25, CD44, and CD69 expression on CD4+ T cells; data not shown).
Continuous thymic output restricts the survival of peripheral CD4+ T cells in MHC class II molecule-expressing mice
Preinjected peripheral CD4+ or CD8+ T cells did not interfere with the survival of a cohort of CD4+ cells transferred into recipient mice expressing MHC class II molecules (Figure 6). By contrast, CD4+ T cells transferred into normal C57BL/6 mice disappeared with time (Figure 5). However, while preinjection can fill the peripheral T-cell compartment of a CD3ϵ-/- mouse, it cannot mimic the daily thymic export of newly generated single-positive T cells. We thus postulated that the failure of a cohort of CD4+ T cells to survive in normal C57BL/6 would be due to their gradual substitution by thymic migrants.
Multiple factors restrict the size of the peripheral CD4+T-cell compartment. (A-B) The peripheral CD4+ T-cell pool is increased in CD8+ T-cell-free mice. (A) Lymph nodes and spleen from 8-week-old male C57BL/6 β2m-/- and C57BL/6 mice were recovered and pooled, and single-cell suspensions were prepared. Absolute numbers of recovered CD4+ TCRhicells were calculated. (B) C57BL/6 mice were injected intraperitoneally with 50 μg of purified anti-CD8 Ab (53-6.7) or with PBS every 2 days for 4 weeks. Absolute numbers of CD4+ TCRhicells are shown at various times during the treatment period. (C) Continuous thymic output restricts the survival of peripheral CD4+ T cells in MHC class II molecule-expressing mice. Seven-week-old C57BL/6 CD45.2 mice were thymectomized or sham thymectomized. Thirty-five days after surgery, the mice were injected with 5 × 106CFSE-labeled lymph node CD4+ T cells from C57BL/6 CD45.1 mice. CFSE fluorescence histograms and absolute numbers of recovered CD45.1 CD4+ TCRhicells are shown 28 days after transfer. (A-C) Data represent mean ± SD.
Multiple factors restrict the size of the peripheral CD4+T-cell compartment. (A-B) The peripheral CD4+ T-cell pool is increased in CD8+ T-cell-free mice. (A) Lymph nodes and spleen from 8-week-old male C57BL/6 β2m-/- and C57BL/6 mice were recovered and pooled, and single-cell suspensions were prepared. Absolute numbers of recovered CD4+ TCRhicells were calculated. (B) C57BL/6 mice were injected intraperitoneally with 50 μg of purified anti-CD8 Ab (53-6.7) or with PBS every 2 days for 4 weeks. Absolute numbers of CD4+ TCRhicells are shown at various times during the treatment period. (C) Continuous thymic output restricts the survival of peripheral CD4+ T cells in MHC class II molecule-expressing mice. Seven-week-old C57BL/6 CD45.2 mice were thymectomized or sham thymectomized. Thirty-five days after surgery, the mice were injected with 5 × 106CFSE-labeled lymph node CD4+ T cells from C57BL/6 CD45.1 mice. CFSE fluorescence histograms and absolute numbers of recovered CD45.1 CD4+ TCRhicells are shown 28 days after transfer. (A-C) Data represent mean ± SD.
To test this possibilty, CD4+ T cells from C57BL/6 CD45.1 mice were transferred into thymectomized and sham-thymectomized C57BL/6 CD45.2 mice. The survival of CD45.1 CD4+ T cells was assessed 28 days after their transfer (Figure 7C). As expected, CD4+ T-cell survival was markedly enhanced in thymectomized mice, suggesting a role of thymic migrants in the continuous renewal of the peripheral CD4+ T-cell compartment. By contrast, the survival of transferred CD4+ T cells in MHC IIΔ/Δ mice was not improved by thymectomy (data not shown).
Discussion
In normal mice, the proliferation of transferred polyclonal CD4+ T cells is quite limited, whereas most CD4+ T cells proliferate after transfer into T-cell-free recipients. In these latter recipients, while most transferred CD4+ T cells undergo a limited number of divisions, some proliferate rapidly and expand strongly (leading to the generation of CFSE- CD4+ T cells), a process recently designated by Min et al as “spontaneous proliferation.”22,23,26 Here we demonstrate that spontaneous proliferation of CD4+ T cells in a lymphopenic environment depends strictly on interactions with MHC molecules—mainly MHC class II molecules in MHC class II-expressing mice and MHC class I molecules in mice lacking MHC class II molecules. This requirement for interactions with MHC molecules may explain why, like antigen-driven proliferation, spontaneous proliferation of naive T cells in a lymphopenic environment is independent of interleukin-7 (IL-7),23 and results in their differentiation into memory cells.17 Indeed, strongly proliferating T cells in lymphopenic hosts share a similar gene-expression profile with conventional memory T cells.27 Although recent data suggest the involvement of enteric bacterial peptides in the spontaneous proliferation of naive T cells,24 a role for self-peptides in this process cannot be ruled out. If this is indeed the case, then spontaneous proliferation would correspond to an autoimmune process, potentially explaining why lymphopenic individuals are at much higher risk of developing certain autoimmune diseases.28-31
While a few clones rapidly proliferate and convert to a memory phenotype, the bulk of CD4+ T cells transferred into lymphopenic hosts (CFSE+ CD4+ T cells) proliferate slowly and retain a naive phenotype. The extent of this proliferation also depends on interactions with MHC class II molecules. Indeed, at all time-points after transfer, we found that CFSE+ CD4+ T cells proliferated more strongly in CD3ϵ-/- mice than in CD3ϵ-/-IIΔ/Δ mice (Figure 1). Nevertheless, contrary to spontaneous proliferation, this slow proliferation of CD4+ T cells was also observed in the complete absence of MHC molecules (Figure 4). Thus, stimuli other than TCR signaling could mediate slow T-cell proliferation in a lymphopenic environment. By studying either polyclonal or monoclonal T cells, several groups have shown that the slow proliferation of T cells in lymphopenic hosts is IL-7 dependent.23,32-35 In lymphopenic hosts, IL-7 would be sufficently abundant to permit slow TCR-independent CD4+ T-cell proliferation. Accordingly, preinjected CD4+ or CD8+ T cells inhibited the slow proliferation of secondarily transferred CD4+ T cells (Figure 6), probably by consuming IL-7, thus limiting its availability for the second cohort of injected T cells.
Regarding the biological significance of this slow cytokinemediated proliferation of T cells in a lymphopenic environment, most researchers consider it to be “homeostatic,” because it would restore the peripheral T-cell compartment. In our hands, although CFSE+ CD4+ T cells cycled slowly, their absolute numbers did not increase with time, suggesting a counterbalancing effect due to death of proliferating cells. Furthermore, this slow proliferation is not required for T cells to maintain their numbers. Indeed, in lymphopenic mice lacking MHC class II molecules, although preinjected CD4+ T cells inhibited the slow proliferation of a second cohort of CD4+ T cells, they did not affect the survival of this cohort (Figure 6). Thus, the slow proliferation of naive T cells in a lymphopenic environment cannot fill the peripheral T-cell compartment and is not required for its maintenance. Slow proliferation of naive T cells in a lymphopenic environment does not therefore appear to be a homeostatic mechanism.
We found that long-term survival of CD4+ T cells did not depend on TCR signaling in lymphopenic mice. Indeed, despite the decrease in ζ phosphorylation of CD4+ T cells observed 2 days after transfer into CD3ϵ-/-IIΔ/Δ mice, the bulk of CD4+ T cells (CFSE+ CD4+ T cells) maintained a constant number for at least 2 months. Thus, the survival of CD4+ T cells relies on stimuli other than self-recognition in a lymphopenic environment. Increased IL-7 availability in lymphopenic mice induces the bulk of naive T cells to proliferate slowly.23,32-35 Perhaps the same cytokine also permits peripheral naive T cells to survive in this context.33,36,37 However, as stated, our competition experiments clearly demonstrated that this slow proliferation is not itself required for naive T cells to maintain their numbers, as it was easier to inhibit the slow proliferation than the survival of CD4+ T cells in a lymphopenic environment.
In both normal mice and MHC IIΔ/Δ mice, transferred CD4+ T cells disappear with time. Nevertheless, the half-life of injected CD4+ T cells was twice as long when recipient mice expressed MHC class II molecules. Thus, self-recognition allows CD4+ T cells to persist for longer in nonlymphopenic mice. Our results conflict with those of Dorfman et al7 and Clarke et al,6 who observed that, in a nonlymphopenic environment, the kinetics of CD4+ T cells disappearance was similar whether or not the recipient mice expressed MHC class II molecules. Both Dorfmann et al7 and Clarke et al7 used Aβ-knock-out mice as MHC class II-deficient mice. However, we have recently shown that in A -/-β mice, the Aα chain and the Eβ chain associate to form a hybrid AαEβ MHC class II molecule.20 In these mice, potential interactions between injected CD4+ T cells and this nonclassical MHC class II molecule might have interfered with the results.
Finally, as previously proposed by Tanchot et al38,39 for naive CD8+ T cells, we found that the disappearance of naive CD4+ T cells in normal C57BL/6 mice was due to their gradual replacement by new CD4+ thymic migrants. Indeed, transferred CD4+ T-cell numbers remained constant in thymectomized normal C57BL/6 mice while, importantly, they still failed to survive in thymectomized MHC IIΔ/Δ mice.
During thymic differentiation, recognition of self-peptide/self-MHC ligands and the resulting activation process (positive selection) are required for immature thymocytes to differentiate into fully mature lymphocytes.40,41 Thymocytes also require signals generated by receptor recognition of self-ligands for survival, maturation, and orientation toward the CD4+ or CD8+ lineage.42-44 Our results demonstrate that naive CD4+ T cells continue to interact with MHC molecules in the periphery, and that such interactions are required for their survival in a nonlymphopenic environment. Thus, in normal mice, intimacy between T cells and “self” is not restricted to the thymus. Our results strongly support a role of self-recognition in the maintenance of the peripheral CD4+ T-cell pool.
Prepublished online as Blood First Edition Paper, March 9, 2006; DOI 10.1182/blood-2006-01-0017.
Supported by a PhD fellowship from Fondation pour la Recherche Médicale (B.M.). This work was supported by a grant from the French Research Ministry and by a grant for young investigators from the French National Research Agency (ANR).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank B. Faideau and A. Le Campion for illuminating discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal