Natural-killer (NK)-cell dysfunction and IFN-γ deficiencies have been associated with increased incidence of both malignancy and infection. The immunologic basis of NK-cell defects in cancer-bearing hosts has not been extensively studied. Here, we demonstrate that multiple lineages of tumors, including thymoma, breast cancer, colon cancer, and melanoma cell lines, interrupt functional maturation during NK-cell development in the bone marrow. The immature NK cells in the periphery of tumor-bearing mice had impaired IFN-γ production but seemingly normal cytotoxicity. T cells are not involved in this NK maturation arrest, because T-cell depletion did not restore NK-cell development. Moreover, the extent of tumor-cell infiltration into the bone marrow does not correlate with defective NK maturation. Interestingly, the defect was associated with a significant reduction in the IL-15Rα+ cells in the non-T, non-NK compartment of bone marrow cells and restored by overexpression of IL-15. Our data demonstrate that tumor growth can impede functional maturation of NK cells, most likely by interrupting the requisite IL-15 signaling pathway. (Blood. 2006;108:246-252)
Introduction
Natural killer (NK) cells are a third subset of lymphocytes and function as part of the innate immune system.1,2 Unlike T and B lymphocytes, NK cells do not use clonally distributed receptors, which alleviates the need for extensive clonal expansion and enables a rapid NK-cell response to infection and malignancy.1,2 In addition, because the complex process of V-D-J recombination and specificity selection demands an extensive time span for T-cell education in the thymus (usually > 3 weeks),3,4 it is less likely that the developmental program of T cells can be adjusted rapidly to make a difference to the T-cell response. In contrast, mature NK cells can be produced from bone marrow in fewer than 8 days.5 An interesting but largely unresolved issue in NK-cell biology is whether their maturation is adversely affected by either infections or cancer.
Mouse NK-cell precursors are found primarily in the bone marrow5 and are characterized as being CD122+ with expression of IL-15Rα, Id2, Gata-3, Ets-1, and the 2B4 markers.6 Because NK-cell production is inhibited by treatments that cause marrow ablation and/or myelosuppression, it has been suggested that the bone marrow is the site for mouse NK-cell development,5 although recent studies by one of us suggest that in humans this process may occur in secondary lymphoid tissue.7 Kim et al8 proposed 5 steps for NK-cell development in mouse bone marrow. According to this model, NK-cell precursors that characterize stage I are CD122+NK1.1-DX5-. The developing NK cell acquires NK1.1, αv, and CD94 in stage II and Ly49 in stage III. NK cells expand primarily at stage IV, which is marked by down-regulation of αv and increased DNA synthesis. Accompanying increased expression of CD11b and CD43, NK cells acquired their full effector function at stage V, although the CD11b “low” NK cells have some capacity to lyse target cells and secrete IFN-γ.
A long-standing clinical observation is the association between defective NK-cell activity and cancer progression. For example, in patients with lung cancer, the advancing stages of disease are significantly associated with reduced NK activity even though the NK cells capable of forming aggregates with tumor cells were apparently normal.9 Decreased NK activity was observed in patients with hereditary colorectal adenocarcinoma10 and in high-risk family members,11 and in healthy individuals from families with a high incidence of breast cancer.12 A significant reduction of lymphokine-activated killer activity was observed in patients with hepatocellular carcinoma, which is reversible after surgical removal of the cancerous tissue.13 In patients with chronic myeloid leukemia, a significant reduction of the absolute number of total NK cells, the relative number of the CD56bright NK subset, and proliferative potential of individual NK cells were all affected.14 Similar defects were also observed in patients with metastatic melanoma.15 Costello et al16 showed that most patients with acute myelocytic leukemia have NK cells with lower levels of NK-cell cytotoxicity receptors and poor lytic activity toward target cells. These data suggest that defective NK cells may contribute to defective immunity against a large variety of cancers.
With the delineation of the developmental pathway for NK-cell maturation, it is of interest to define the effect of tumor burden on NK development and activation. In this study, we analyzed the effect of multiple lineages of tumors on in vivo NK-cell development. Our data demonstrate that distant tumor growth renders defective development of NK cells that was mapped to the final stage, namely acquisition of effector function. Interestingly, the defect is reversed by overexpression of IL-15, but not by T-cell depletion. These results reveal a novel mechanism for down-regulation of innate immunity and a novel function of IL-15 in the functional maturation of NK cells.
Materials and methods
Mice
C57Bl/6 and Balb/c mice were purchased from Charles River Laboratories (Wilmington, MA) under contract from the National Cancer Institute. B6 PLThy1<a>/cy and C57Bl/6-LySU-Pep3B were purchased from the Jackson Laboratory (Bar Harbor, ME). IL-15Tg mice have been described.17 All mice were housed in the University Laboratory Animal Facility at The Ohio State University under specific pathogen-free conditions. All protocols involving animal work have been approved by the institutional laboratory animal care and use committee.
Antibodies
The following fluorochrome-conjugated antibodies were purchased from eBioscience (San Diego, CA): fluorescein isothiocyanate (FITC)- or allophycocyanin (APC)-conjugated CD3ϵ (clone 145-2C11), APC-anti-NK1.1 (clone PK 136), phycoerythrin (PE)-anti CD11b (clone M1/70), PE-anti-CD51 (clone RMV-7), PE-anti-CD94 (clone 18d3), PE-anti-mouse NK cell (Ly-49 C/I/F/H; clone 14B11), FITC-anti-IFN-γ (clone XMG1.2), and APC-anti-CD90.2 (clone 53.2.1). The following fluorochrome-conjugated antibodies were purchased from BD Pharmingen (San Diego, CA): peridinin chlorophyll protein Cy5.5 (PercpCy5.5)-conjugated anti-NK1.1 (clone PK136); PercpCy5.5-CD11b (clone M1/70), PE-anti-Ly49A+D (clone 12A8), PE-anti-CD45.1 (clone A20), PE-anti-CD122 (clone TM-β1), FITC-anti-CD49b/Pan-NK cells (clone DX5), FITC-anti-BrdU (clone 3D4), and FITC-IgG1 isotype control (clone MOPC-21). Biotinylated anti-mouse IL-15Rα was obtained from R&D System (Minne-apolis, MN). The monoclonal antibodies GK1.5 and 2.4.3 were purified through protein G column.
Tumors
Thymoma EL4, T-leukemia cell line RMA-S, melanoma B16F1, and colon cancer cell line MC38 are syngeneic to C57Bl/6j mice, whereas breast cancer cell line TSA and 4T1 are syngeneic to Balb/c mice. EL4 and RMA were grown in RPMI 1640 medium supplemented with 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 4 mM l-glutamine. B16F1, MC38, 4T1, and TSA were all grown in DMEM medium supplemented with 5% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 4 mM l-glutamine.
Establishment of subcutaneous tumor
Syngeneic mice were subcutaneously injected with EL4 (5 × 106 cells), B16F1 (1 × 104 cells), MC38 (5 × 105 cells), 4T1 (5 × 105 cells), or TSA (5 × 105 cells) viable tumor cells. Mice were monitored every 2 to 3 days to evaluate tumor growth, and subcutaneous tumors were measured with caliper along perpendicular axes of the tumor. Mice were killed when tumors reached a size of 20 mm.2
Cell preparation and flow cytometry
Single-cell suspensions were prepared from bone marrow and spleen and were depleted of red blood cells. For flow cytometry, cells were initially incubated with 2.4G2 supernatant to block nonspecific antibody binding. Cells were then stained with 4-color combinations of indicated fluorochrome-conjugated monoclonal antibodies. Stained cells were fixed and analyzed with a FACSCalibur (Becton Dickinson, San Jose, CA).
Stimulation of IFN-γ and intracellular staining
To induce IFN-γ production by NK cells in vivo, control and tumor-bearing mice were intraperitoneally injected with 200 μg LPS. After 6 hours mice were killed, and single-cell suspensions were prepared from spleens. Splenocytes were stained for surface markers, fixed, permeabilized, and then stained for IFN-γ according to the manufacturer's instructions for the Cytofix/Ctyoperm kit (BD Pharmingen).
Cytotoxic assay
NK-cell cytotoxicity was determined by the lysis of the NK-sensitive cell line Yac-1 in a standard 51Cr-release assay. Target Yac-1 cells were labeled with 51Cr for 1 hour at 37°C. Labeled Yac-1 cells were incubated with splenocytes from control and tumor-bearing mice at the indicated ratios for 6 hours. Supernatants were collected, and the percentage of specific lysis was calculated by using the following formula: experimental - minimum/maximum - minimum × 100.
Detection of proliferating cells
Control or tumor-bearing mice were intraperitoneally injected with 1 mg BrdU. After 3 hours, mice were killed, and single-cell suspensions were prepared from spleen and bone marrow. Cells were blocked with 2.4G2 supernatant and surface stained for NK cells. The BrdU Flow kit (BD Pharmingen) was then used to fix, permeabilize, DNAse treat, and stain for BrdU according to the manufacturer's instructions.
Adoptive transfer
Bone marrow was prepared from 10 to 15 C57BL/6-LySU-Pep3B mice and stained with CD3, CD122, NK1.1, and CD11b. CD122+ cells were positively selected through a magnetic cell sorting column after which NK1.1+CD11b- cells were sorted. The purity of the NK1.1+CD11b- was greater than 98%. Sorted NK1.1+CD11blo cells (2.5 × 104) were intravenously injected into C57Bl/6j mice that were previously injected 7 days earlier with either PBS or EL4. After 14 days, cells were isolated from the spleen of recipient mice for flow cytometric analysis.
In vivo T-cell depletion
The antibodies GK1.5 and 2.4.3 were used to deplete CD4 and CD8 T cells, respectively. These antibodies were intraperitoneally administered at a dose of 500 μg/mouse on day -3, 0, and +3 of EL4 or PBS injection.
Results
Tumor growth selectively blocks maturation of NK cells in the bone marrow
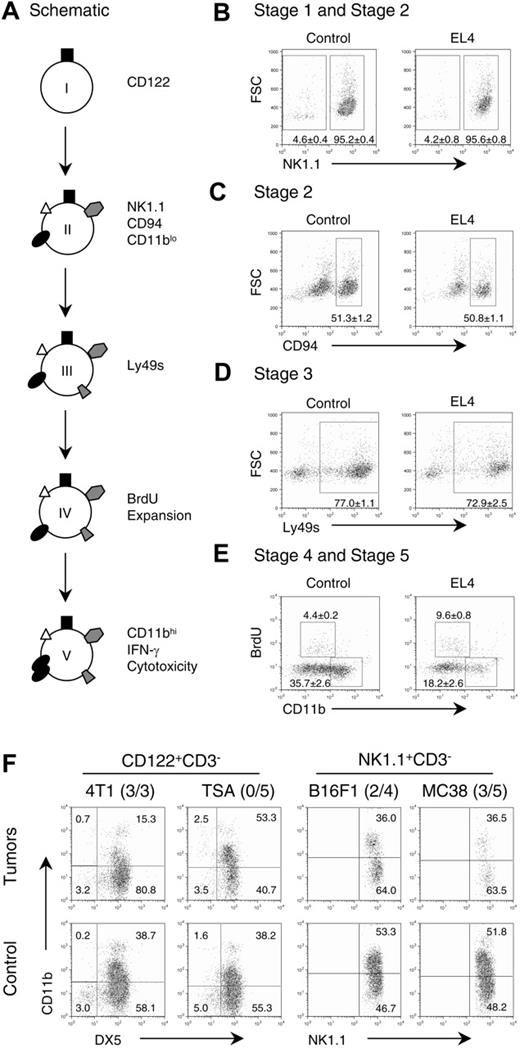
The developmental pathway of NK cells in the bone marrow is delineated into 5 stages by Kim et al8 and recapitulated in Figure 1A. To determine whether the defects in NK cells in the periphery could be caused by tumor-induced developmental arrest, we systematically compared NK-cell development in the bone marrow of control and tumor-bearing mice. Cells of NK lineage were identified by their CD3-CD122+ phenotype. As shown in Figure 1B, the expression of NK1.1 is present on 95% of CD122+ cells from control and tumor-bearing mice. Consistent with the expression of NK1.1, CD94 is also comparable between control and tumor-bearing mice, indicating that the transition from stage I to II is unaffected (Figure 1C). To determine whether the acquisition of Ly49 surface expression is abrogated by tumor growth, we analyzed LY49 expression with a cocktail of antibodies, which in combination recognizes, Ly49A, Ly49C, Ly49D, Ly49F, Ly49H, and Ly49I. As shown in Figure 1D, the overall percentages are quite similar between the tumor-bearing mice and control mice. A defining feature of stage IV is the proliferation of immature NK cells, which can be measured by DNA replication. We pulsed the tumor-bearing and control mice with the nucleotide analog BrdU. Three hours later, bone marrow NK1.1+CD3- cells were gated and analyzed for their expression of CD11b and BrdU incorporation. As shown in Figure 1E, substantial BrdU+ cells were observed among the cells that have intermediate CD11b surface expression, which is consistent with the data from Kim et al.8 Surprisingly, a slight increase of BrdU incorporation was observed in the tumor-bearing mice. However, their total number of dividing NK cells was unchanged because the total NK cells was reduced in this model (data not shown). These data demonstrated that tumor growth did not blunt proliferation of immature NK cells. Finally, up-regulation of CD11b (CD11bhi-) is an important marker for functional maturation of NK cells.8 In Figure 1E, the NK-cell population in the bone marrow of tumor-bearing mice consists of drastically reduced CD11bhi cells compared with control. The reduction of CD11bhi was not due to increased apoptosis, because the Annexin V+ NK cells were not elevated in tumor-bearing mice (data not shown). These results demonstrate that developing NK cells in tumor-bearing mice have a block in transition from stage IV to stage V, which impairs their functional maturation.
Tumor growth inhibits NK-cell development at stage IV. (A) Schematic depiction of the stages of NK-cell differentiation in vivo. (B-E) The effect of tumor growth on the development of NK cells in the bone marrow. Bone marrow cells were obtained from control and EL4 tumor-bearing mice and stained with CD3 and CD122 (A,B,D) or CD3 and NK1.1 (C,E) in conjunction with other markers. The CD122+CD3- cells were evaluated for expression of NK1.1 (B); CD94 (C); Ly49A, Ly49C, Ly49D, Ly49H, and Ly49I (D), as well as BrdU and CD11b (E). The numbers shown in the panels are mean ± SEM (n = 4) of the percentage of cells in the gates. The decreases of CD11b expression (P ≤ .001) and increase in proliferation (P ≤ .001) in tumor-bearing mice were highly significant. (F) Multiple lineages of syngeneic tumors caused developmental defects of NK cells in the bone marrow. NK cells from syngenic mice bearing mammary tumors (4T1 or TSA), melanoma (B16F1), or colon adenocarcinoma (MC38) were evaluated for NK-cell maturation. NK cells were identified based on their phenotypes, that is, CD122+CD3- in BALB/c mice and NK1.1+CD3- in C57BL/6 mice. Fluorescence-activated cell sorting (FACS) profiles depict CD11b expression among NK cells. The numbers of mice with indicated defects over the number of mice tested are shown next to the tumor cells used.
Tumor growth inhibits NK-cell development at stage IV. (A) Schematic depiction of the stages of NK-cell differentiation in vivo. (B-E) The effect of tumor growth on the development of NK cells in the bone marrow. Bone marrow cells were obtained from control and EL4 tumor-bearing mice and stained with CD3 and CD122 (A,B,D) or CD3 and NK1.1 (C,E) in conjunction with other markers. The CD122+CD3- cells were evaluated for expression of NK1.1 (B); CD94 (C); Ly49A, Ly49C, Ly49D, Ly49H, and Ly49I (D), as well as BrdU and CD11b (E). The numbers shown in the panels are mean ± SEM (n = 4) of the percentage of cells in the gates. The decreases of CD11b expression (P ≤ .001) and increase in proliferation (P ≤ .001) in tumor-bearing mice were highly significant. (F) Multiple lineages of syngeneic tumors caused developmental defects of NK cells in the bone marrow. NK cells from syngenic mice bearing mammary tumors (4T1 or TSA), melanoma (B16F1), or colon adenocarcinoma (MC38) were evaluated for NK-cell maturation. NK cells were identified based on their phenotypes, that is, CD122+CD3- in BALB/c mice and NK1.1+CD3- in C57BL/6 mice. Fluorescence-activated cell sorting (FACS) profiles depict CD11b expression among NK cells. The numbers of mice with indicated defects over the number of mice tested are shown next to the tumor cells used.
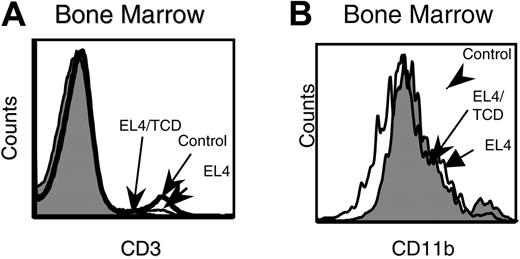
T-cell depletion does not reverse the NK-cell developmental defect in tumor-bearing mice. CD4 and CD8 T cells were depleted by the administration of GK1.5 and 2.4.3 on day -3, 0, and +3 of tumor-cell challenge. Control PBS-treated, EL4-challenged, and T-cell-depleted (TCD) EL4-challenged mice were evaluated for NK-cell differentiation. (A) Effects of GK1.5 and 2.4.3 on T-cell depletion in bone marrow. (B) CD11b expression on NK1.1+CD3- bone marrow cells. Data are representative of 2 separate experiments.
T-cell depletion does not reverse the NK-cell developmental defect in tumor-bearing mice. CD4 and CD8 T cells were depleted by the administration of GK1.5 and 2.4.3 on day -3, 0, and +3 of tumor-cell challenge. Control PBS-treated, EL4-challenged, and T-cell-depleted (TCD) EL4-challenged mice were evaluated for NK-cell differentiation. (A) Effects of GK1.5 and 2.4.3 on T-cell depletion in bone marrow. (B) CD11b expression on NK1.1+CD3- bone marrow cells. Data are representative of 2 separate experiments.
To test whether the developmental blockade of NK-cell activation is a general phenomenon, we challenged 2 strains of mice with 4 syngeneic tumor cell lines and compared the phenotype of their NK cells from the tumor-bearing mice with that from control mice. In the BALB/c mice, 1 of the 2 breast cancer cell lines, 4T1, substantially reduced the up-regulation of CD11b (Figure 1F). In the C57BL/6j mice, both melanoma cell line B16 and colon cancer cell line MC38 blocked up-regulation of CD11b on NK cells. Therefore, although tumor blockade of NK-cell maturation is not universal, it is a general phenomenon that can be documented with multiple tumor cell lines.
Developmental blockade of NK-cell maturation is not mediated by T cells and does not require direct contact between immature NK cells and tumor cells
Previous studies have revealed T-cell-dependent reduction of NK-cell activity in LCMV-infected mice.18 To determine whether T cells are involved in suppressing NK development, we depleted CD4 and CD8 T cells prior to tumor challenge. The accumulation of CD11blo cells in the bone marrow of tumor-bearing hosts was unaffected by effective T-cell depletion (Figure 2). These results demonstrate that tumor-induced blockade of NK maturation is independent of host T cells.
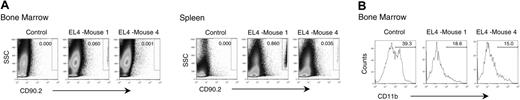
An important issue is whether direct contact between tumor cells and immature NK cells is required to block NK-cell maturation. Because the tumor cells were injected into the flank, they would need to metastasize to the bone marrow to come in contact with developing NK cells. We therefore determined whether tumor metastasis is a prerequisite to the developmental block of NK cells. By using B6 PL Thy1a/cy congenic mice, we were able to distinguish EL4 tumors from host cells. Tumor cells were therefore subcutaneously injected into the Thy1a congenic mice, and 3 weeks after injection the mice were killed and evaluated for tumor metastasis and inhibition of NK-cell development. Our analysis revealed significant differences in the migration of tumor cells to the bone marrow, although essentially all mice tested at this point had EL4 cells in the spleen (Figure 3A). Interestingly, the blockade of NK development was at least as severe in the mice that had barely detectable tumor cells in the bone marrow as those that had a 60-fold higher number of tumor cells in the bone marrow (Figure 3B). Thus, tumor metastasis is unlikely a prerequisite for blocking NK development.
Tumor metastasis into bone marrow is not responsible for developmental defects of NK cells. Congenic mice (CD90.1) were injected with EL4 (CD90.2) or PBS. Mice were killed when tumors reached an average diameter of 20 mm. Box in the top right of each histogram indicates the tumor-cell gates. (A) Splenocytes and bone marrow cells were evaluated for the presence of tumor. (B) Expression of CD11b on NK1.1+CD3- cells. Data from 2 representative EL4 tumor-bearing mice, called EL4-1 and EL4-4, are presented.
Tumor metastasis into bone marrow is not responsible for developmental defects of NK cells. Congenic mice (CD90.1) were injected with EL4 (CD90.2) or PBS. Mice were killed when tumors reached an average diameter of 20 mm. Box in the top right of each histogram indicates the tumor-cell gates. (A) Splenocytes and bone marrow cells were evaluated for the presence of tumor. (B) Expression of CD11b on NK1.1+CD3- cells. Data from 2 representative EL4 tumor-bearing mice, called EL4-1 and EL4-4, are presented.
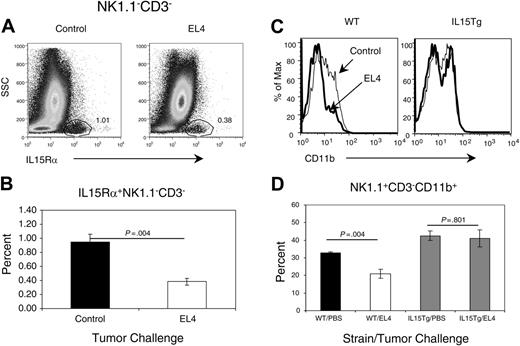
Defective IL-15 signaling contributes to NK defect. (A-B) Tumor growth reduces IL-15Rαhi cells in the bone marrow. Bone marrow cells were isolated from the control PBS-treated or tumor-bearing mice at 3 weeks after tumor-cell challenge and stained with either anti-IL-15Rα antibody or isotype control. (A) Representative profile IL-15Rα expression. The number in the panels shows the percentage of cells in the IL-15Rαhi-cell gate. (B) Summary data on the percentage of IL-15Rαhi cells. A significant difference was observed between tumor-bearing and control mice. (C-D) Transgenic overexpression of IL-15 over-comes NK-cell differentiation defect. IL-15Tg and wild-type littermate controls were injected with EL4 or PBS. Four weeks later, the mice were killed, and bone marrow NK cells were analyzed for CD11b expression. Data shown in panel C are representative FACS profiles of gated NK1.1+CD3- NK cells of 3 independent experiments, whereas those in panel D are means and SEM of the percentage of reduction of CD11bhi cells in tumor-bearing mice. Student t test reveals significant difference in the percentage of CD11bhi cells in non-transgenic littermates.
Defective IL-15 signaling contributes to NK defect. (A-B) Tumor growth reduces IL-15Rαhi cells in the bone marrow. Bone marrow cells were isolated from the control PBS-treated or tumor-bearing mice at 3 weeks after tumor-cell challenge and stained with either anti-IL-15Rα antibody or isotype control. (A) Representative profile IL-15Rα expression. The number in the panels shows the percentage of cells in the IL-15Rαhi-cell gate. (B) Summary data on the percentage of IL-15Rαhi cells. A significant difference was observed between tumor-bearing and control mice. (C-D) Transgenic overexpression of IL-15 over-comes NK-cell differentiation defect. IL-15Tg and wild-type littermate controls were injected with EL4 or PBS. Four weeks later, the mice were killed, and bone marrow NK cells were analyzed for CD11b expression. Data shown in panel C are representative FACS profiles of gated NK1.1+CD3- NK cells of 3 independent experiments, whereas those in panel D are means and SEM of the percentage of reduction of CD11bhi cells in tumor-bearing mice. Student t test reveals significant difference in the percentage of CD11bhi cells in non-transgenic littermates.
Overexpression of IL-15 overcomes developmental block of immature NK cells
Because direct contact with tumor cells is not responsible for the defective NK-cell development, we postulated that such a defect may involve the interruption of a requisite cytokine signaling pathway. IL-15 is an essential growth factor for the development and survival of NK cells.19-22 Under physiologic conditions, IL-15 must bind to its high-affinity IL-15Rα and activate NK cells by a trans-signaling mechanism.23-25 As such, IL-15Rα expression on non-T, non-NK bone marrow stroma cells is generally regarded as the limiting factor for IL-15 signaling in vivo. We therefore compared the levels of IL-15Rα among the bone marrow stroma cells in control and tumor-bearing mice. As shown in Figure 4A, a distinct population of CD3-, NK1.1-IL-15Rα+ cells can be found in the bone marrow of both control and tumor-bearing mice. However, this subset is reduced by nearly 3-fold in tumor-bearing mice (Figure 4B). Thus, tumor growth resulted in significant reduction of IL-15Rα expression.
To determine whether an IL-15-signaling defect alone explains the block in NK-cell maturation, we tested whether transgenic overexpression of IL-1517 may overcome the NK defect in tumor-bearing mice by comparing the phenotype of NK cells in IL-15 transgenic mice with their non-transgenic (WT) littermates. As shown in Figure 4C-D, a significant reduction of CD11bhi NK cells was observed in the bone marrow of WT littermates as a result of tumor growth. In contrast, the expression of CD11b was essentially identical in tumor-challenged IL-15 transgenic mice to those in non-tumor-bearing mice. These results demonstrated that defective NK development could be overcome with ectopic expression of IL-15.
EL4 impedes IFN-γ production but not cellular cytotoxicity by NK cells
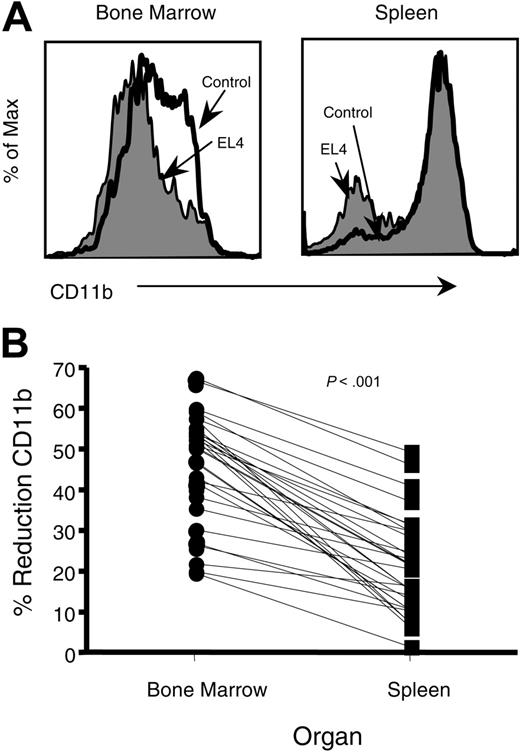
An important issue is whether the defective NK-cell development leads to defective NK-cell function in the periphery. To address this issue, we compared the expression of CD11b on NK cells found in the bone marrow with those of the spleen (Figure 5A). In tumor-bearing mice, the percentage of CD11bhi-expressing NK cells had a mean reduction of 46.6% in the bone marrow when compared with mice without tumor, whereas the average decrease in CD11bhi NK cells in the spleen was 21.4% (Figure 5B).
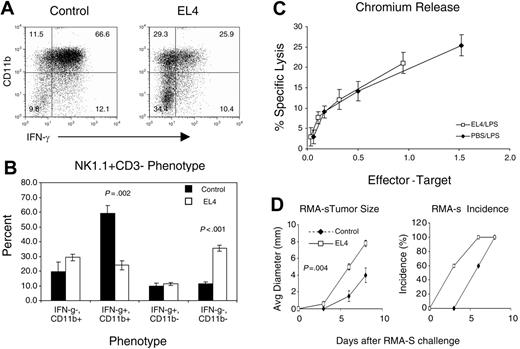
Previous studies have reported that NK cells with high levels of CD11b are more mature, have increased interferon-γ (IFN-γ) production, and cellular cytotoxicity.8 To determine whether EL4 modifies NK-cell function, control and tumor-bearing mice were injected with LPS. NK cells were monitored for intracellular IFN-γ and cellular cytotoxicity 6 hours after LPS stimulation in vivo. In control mice, the overwhelming majority of NK cells expressed high levels of CD11b, which rapidly synthesize IFN-γ in response to Toll-receptor agonists such as LPS (Figure 6A). In mice with large tumors, the percentage of CD11bhi and IFN-γ-producing NK cells are reduced (Figure 6A). Furthermore, the percentage of IFN-γ-producing CD11blow NK cells did not change, even though there was a 2- to 3-fold increase in that population (Figure 6B).
Reduction of CD11bhicells is more prevalent in bone marrow than in spleen. (A) Representative CD11b profiles of NK (NK1.1+CD3-) cells from bone marrow and spleen of tumor-bearing mice. (B) The percentage of reduction of CD11bhi cells in EL4-bearing mice was calculated according to the following formula: CD11bhi control - CD11bhi tumor / CD11bhi control × 100. The percentage of reduction was then compared between bone marrow and spleens and found highly significant, using a paired t test.
Reduction of CD11bhicells is more prevalent in bone marrow than in spleen. (A) Representative CD11b profiles of NK (NK1.1+CD3-) cells from bone marrow and spleen of tumor-bearing mice. (B) The percentage of reduction of CD11bhi cells in EL4-bearing mice was calculated according to the following formula: CD11bhi control - CD11bhi tumor / CD11bhi control × 100. The percentage of reduction was then compared between bone marrow and spleens and found highly significant, using a paired t test.
NK cells have impaired IFN-γ production but normal cytotoxicity in a tumor-bearing host. Splenocytes from control and EL4-bearing mice that were injected with LPS were tested for IFN-γ production and cytotoxicity. (A) NK1.1+CD3- splenocytes were evaluated for CD11b expression and IFN-γ production. (B) The mean ± SEM of the NK-cell phenotype. The NK-cell populations CD11b+IFN-γ+ (P ≤ .002) and CD11b-IFN-γ- (P < .001) were significantly decreased and increased, respectively, in tumor-bearing mice compared with controls. (C) Splenocytes were tested for cytotoxic potential against the NK-sensitive target Yac-1. The E/T ratios were adjusted based on the percentage of NK cells in the spleen. The experiment is representative of 3 separate experiments. (D) EL4 tumor-bearing mice had diminished NK immunity toward tumor challenge. Control and EL4 tumor-bearing mice were challenged with 2.5 × 106 RMA-S cells subcutaneously injected at day 13 after EL4 challenge (n = 5). The sizes (left) and incidence (right) of RMA-S tumor are measured by physical examination. Size data shown are the mean ± SEM of tumor diameters (P = .004).
NK cells have impaired IFN-γ production but normal cytotoxicity in a tumor-bearing host. Splenocytes from control and EL4-bearing mice that were injected with LPS were tested for IFN-γ production and cytotoxicity. (A) NK1.1+CD3- splenocytes were evaluated for CD11b expression and IFN-γ production. (B) The mean ± SEM of the NK-cell phenotype. The NK-cell populations CD11b+IFN-γ+ (P ≤ .002) and CD11b-IFN-γ- (P < .001) were significantly decreased and increased, respectively, in tumor-bearing mice compared with controls. (C) Splenocytes were tested for cytotoxic potential against the NK-sensitive target Yac-1. The E/T ratios were adjusted based on the percentage of NK cells in the spleen. The experiment is representative of 3 separate experiments. (D) EL4 tumor-bearing mice had diminished NK immunity toward tumor challenge. Control and EL4 tumor-bearing mice were challenged with 2.5 × 106 RMA-S cells subcutaneously injected at day 13 after EL4 challenge (n = 5). The sizes (left) and incidence (right) of RMA-S tumor are measured by physical examination. Size data shown are the mean ± SEM of tumor diameters (P = .004).
Nevertheless, the defects in NK-cell function were not universal. When spleen cells from tumor-bearing mice were stimulated in vitro with PMA and ionomycin, the numbers of IFN-γ-producing NK cells were drastically reduced. In contrast, in response to IL-2 + IL-12 or anti-NK1.1 antibody, the number of IFN-γ-producing NK cells was not significantly affected (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Moreover, NK-cellular cytotoxicity was normal (Figure 6C).
To determine whether NK-mediated tumor immunity is affected in the tumor-bearing mice, we challenged EL4 tumor-bearing mice with RMA-S tumor cells that lack MHC class I and rejected by syngeneic NK cells in vivo.26 As shown in Figure 6D, the RMA-S cells grew significantly faster in the tumor-bearing host. Thus, tumor growth reduces the in vivo function of NK cells.
Defective differentiation of immature NK cells in the tumor-bearing mice
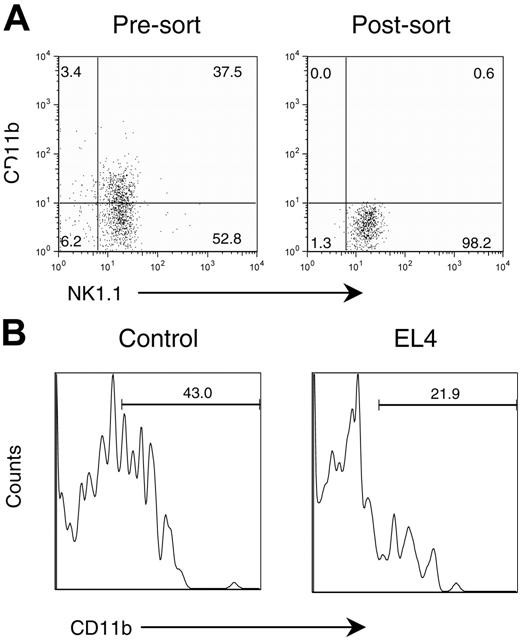
To directly demonstrate that tumor growth can block maturation of NK cells, we purified the immature NK cells from the bone marrow of CD45.1 mice based on their lack of cell-surface CD11b by flow cytometric sorting. As shown in Figure 7A, the purified CD3-NK1.1+ cells consisted of approximately 40% of the CD11b+ subset. After sorting, we obtained uniform CD11b-NK1.1+ cells. The immature CD45.1 NK cells were injected into either control PBS-treated or tumor-bearing mice. Two weeks later, the mice were killed, and the phenotypes of CD45.1+ donor cells were analyzed by flow cytometry. As shown in Figure 7B, about 40% of the cells injected into the PBS control mice up-regulated CD11b. In tumor-bearing mice, however, only about 20% of the immature NK cells expressed CD11b (Figure 7B). These results illustrate a direct effect of tumor growth on the maturation of NK cells.
Discussion
Much like adaptive immunity, NK cell-mediated immunity has distinct phases of development and effector function. A largely unresolved issue in both branches of the immune response is how these 2 processes are connected. In this study, we presented several lines of evidence that distant tumor growth has a significant adverse effect on the maturation of NK cells in the bone marrow, which is regarded as the central lymphoid organ for the development of murine NK cells.
We demonstrated that NK cells from mice challenged with multiple lineages of tumor cells exhibit a CD11blo phenotype. Adoptive transfer of CD11b- NK progenitor cells into tumor-bearing mice directly demonstrated a maturation arrest by tumors. We have analyzed the developmental defects of NK cells caused by the EL4 tumor cell line based on the scheme proposed by Kim et al.8 Our systematic analysis of NK development revealed that a developmental block resides in the last step of NK-cell maturation. Although this effect is not universal, it is clearly demonstrated in 4 of 5 tumor cell lines of different origin. Thus, the developmental arrest reported here is likely to be a general feature of tumor growth.
Tumors impede the maturation of adoptively transferred CD11b-NK cells. Congenic (CD45.1) immature NK cells (NK1.1+CD11b-) from bone marrow were adoptively transferred into control and EL4-bearing mice (CD45.2). Two weeks after the adoptive transfer, splenocytes were analyzed by flow cytometry. (A) Expression of CD11b and NK1.1 in presorted and postsorted samples. The postsorted NK1.1+CD11b- cells were used for adoptive transfer. (B) Histogram of adoptively transferred NK cells in PBS-treated host or in tumor-bearing host. The profiles depict the expression of CD11b among the CD45.1+NK1.1+ cells and are representative of data from 2 separate experiments.
Tumors impede the maturation of adoptively transferred CD11b-NK cells. Congenic (CD45.1) immature NK cells (NK1.1+CD11b-) from bone marrow were adoptively transferred into control and EL4-bearing mice (CD45.2). Two weeks after the adoptive transfer, splenocytes were analyzed by flow cytometry. (A) Expression of CD11b and NK1.1 in presorted and postsorted samples. The postsorted NK1.1+CD11b- cells were used for adoptive transfer. (B) Histogram of adoptively transferred NK cells in PBS-treated host or in tumor-bearing host. The profiles depict the expression of CD11b among the CD45.1+NK1.1+ cells and are representative of data from 2 separate experiments.
Interestingly, direct contact between tumor cells and immature NK cells was not necessary for the developmental block, because comparable blockade was observed regardless of whether tumor cells have migrated into the bone marrow. This observation suggested that abnormal environmental factors, perhaps a defect related to basal level of cytokines, may be responsible for the block. IL-15 is essential for the development and survival for NK cells. IL-15-deficient mice show drastically reduced number of NK cells,27 and those with NK markers show specific defects in Ly49 expression.28 We observed a significant down-regulation of IL-15Rα+ cells among bone marrow stroma cells. Because IL-15Rα is a necessary component for IL-15 signaling under physiologic condition,23,24 it is most likely that IL-15 signaling is reduced in vivo regardless of whether IL-15 is reduced in the tumor-bearing mice. Nevertheless, the NK-cell defect in tumor-bearing mice is less severe than that in IL-15-deficient mice. This can be explained by the fact that the IL-15-signaling pathway is compromised but not eliminated.
To determine whether the IL-15-signaling defect is responsible for accumulation of immature NK cells, we tested whether NK-cell development can be rescued by transgenic overexpression of IL-15. We demonstrated that transgenic expression of high-level IL-15 rescued NK maturation in tumor-bearing hosts. The precise mechanism by which high lL-15 levels compensate for the IL-15Rα defect is not fully understood at this point, but may involve direct signaling of intermediate affinity IL-15Rβ-γ complex. Our data not only reveal IL-15 signaling as the critical checkpoint for tumor evasion of NK recognition, but also demonstrate a new role for IL-15 in the last steps of NK-cell maturation in the bone marrow. Consistent with this hypothesis, Vosshenrich et al29 showed that the small number of NK cells found in IL-15-deficient mice have a phenotype remarkably similar to what we have observed in the tumor-bearing mice.
The molecular basis for the reduced IL-15Rα expression is unclear at this stage. Because the tumor cells cause NK defects without physically contacting NK cells, it is likely that tumor cells may produce soluble factors to down-regulate IL-15Rα. TGFβ is a likely candidate because it is known to down-regulate GATA-3 and GATA-3-deficient bone marrow cells show a CD11blo phenotype among NK cells.30 However, neutralizing anti-TGFβ antibody in vivo failed to rescue NK-cell maturation (data not shown).
Perhaps because an overall change of NK-cell phenotype requires replacement of preexisting NK cells, we have observed that such an overall replacement is discernible only after the tumor has reached a large size. Of the large number of mice we have evaluated, all those that show NK defects in the spleen have shown a greater defect in bone marrow NK cells. These data are consistent with a model in which the phenotype of NK cells in the spleen is a reflection of their defective maturation in the bone marrow.
Kim et al8 have demonstrated that up-regulation of CD11b associates with increased IFN-γ production and increased cytotoxicity against NK targets. However, our data showed that in tumor-bearing mice the defective NK maturation leads to reduced IFN-γ production but normal NK-mediated cytotoxicity. This is consistent with the reports that in interferon regulatory factor-2-deficient mice, NK cells have a CD11blo phenotype in the bone marrow, deficient IFN-γ production but normal cytotoxicity.31 Because IFN-γ production by NK cells plays an important role in NK-mediated antitumor innate immunity, our data demonstrate that tumors may evade innate immunity by blocking maturation of NK cells in the bone marrow.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-11-4535.
Supported by National Institutes of Health grants P01CA95426, R01 CA58033, and T32CA090223.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ou Li for technical assistance and Lynde Shaw for editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal