Immune tolerance is a central mechanism counteracting tumor-specific immunity and preventing effective anticancer immunotherapy. Induction of tolerance requires a specific environment in which tolerogenic dendritic cells (DCs) play an essential role deviating the immune response away from effective immunity. It was recently shown that maturation of DCs in the presence of PGE2 results in upregulation of indoleamine 2,3-dioxygenase (IDO) providing a potential mechanism for the development of DC-mediated Tcell tolerance. Here, we extend these findings, demonstrating a concomitant induction of IDO and secretion of soluble CD25 after DC maturation in the presence of PGE2. While maturation of DCs induced IDO expression on transcriptional level, only integration of PGE2 signaling led to up-regulation of functional IDO protein as well as significant expression of cell-surface and soluble CD25 protein. As a consequence, T-cell proliferation and cytokine production were significantly inhibited, which was mediated mainly by IDO-induced tryptophan depletion. Of importance, we demonstrate that different carcinoma entities associated with elevated levels of PGE2 coexpress CD25 and IDO in peritumoral dendritic cells, suggesting that PGE2 might influence IDO expression in human DCs in the tumor environment. We therefore suggest PGE2 to be a mediator of early events during induction of immune tolerance in cancer. (Blood. 2006;108:228-237)
Introduction
Regularly, tumors induce a state of immunologic unresponsiveness or tolerance toward tumor-associated antigens.1 Under these circumstances, the tumor mostly evades an immune attack, which can naturally occur or might be induced by specific immunotherapy. Early on, the lack of costimulatory molecules on tumor cells has been suggested as a potential mechanism, and indeed most tumors and tumor-infiltrating antigen-presenting cells (APCs) lack the expression of costimulatory molecules.2,3 In addition, overexpression of soluble factors by tumor and stroma cells including TGFβ,4,5 IL10,6,7 VEGF,8 or PGE29-11 is also causing immune suppression particularly by interfering with the adaptive immune response. While tolerance and immunosuppression are clearly established as the immunologic consequences of these tumor-derived factors, many of the underlying molecular mechanisms remain to be defined.
It is now well appreciated that dendritic cells (DCs) not only induce immunity but also play a major role during the induction of T-cell tolerance, particularly in cancer patients.12 DCs are not only reduced in numbers especially in proximity of the cancer,13-15 but are also characterized by an altered maturation phenotype,1,16 which—at least in part—can be induced by factors such as IL10 or VEGF in maturing DCs in vitro.1,13,17 Some early indications of the molecular mechanisms by which these factors might lead to the generation of tolerogenic DCs have recently been reported.12 For example, VEGF can induce expression of STAT3 within the tumor cell, which in return reduces the secretion of proinflammatory factors such as TNFα, IFNβ, or CCL5, thereby inhibiting DC maturation.18
More recently, the expression of indoleamine 2,3-dioxygenase (gene symbol INDO coding for IDO)19,20 in cancer cells has been associated with the induction of T-cell tolerance.21,22 IDO plays an essential role during degradation of the amino acid tryptophan23 and has been described as a negative protective regulator in autoimmune disorders and mammalian gestation.24 Aberrant expression of IDO in tumor cells was shown to be induced by mutations within the tumor suppressor gene Bin1.25 In murine models, expression of IDO was detected in DCs within tumor-draining lymph nodes.26 In fact, within the immune system, IDO expression seems to be most important in DCs.27-29 Induction of T-cell tolerance by IDO-expressing DCs might be mediated by direct effects on T cells,30-32 indirect effects via changes in APC function,33 or bystander suppression.26 While the inhibitory effect of IDO-expressing DCs on T cells has already been suggested, the signals responsible for the induction of IDO, particularly in the context of tumor-associated DCs, are currently under intense investigation. Several modes of DC stimulation have been established leading to induction of IDO expression and function (eg, stimulation by LPS and CTLA4/CD28; co-incubation with IL10, IFNγ, and estrogens; or signaling via the high-affinity IgE receptor).19,27,31,34-37 It has been speculated that factors other than those currently known to induce IDO are involved in up-regulation and activation of IDO in DCs in this context.19
Most recently, PGE2 was described to up-regulate functional IDO during DC maturation.38 In fact, PGE2 qualifies as a potential candidate for the induction of tolerogenic DCs in a tumor environment, since many tumor entities are described to be associated with elevated levels of PGE2, and an increased production of PGE2 by tumors such as non-small cell lung cancer (NSCLC) or glioma was recently found to be associated with the induction of regulatory T cells39-41 and T-cell inhibition.42 PGE2 plays an essential role during carcinogenesis of tumors that are associated with chronic inflammatory responses,43-45 and deletion of the prostaglandin receptor EP2 leads to reduced carcinogenesis and an enhanced antitumor immunity by altering DC function.46 PGE2 is generated from arachidonic acid by cyclooxygenases (COXs) and prostaglandin E synthase,47 and the synthesis of PGE2 can efficiently be blocked by nonsteroidal anti-inflammatory drugs (NSAIDs), in particular cyclooxygenase-2 (COX-2) inhibitors. These drugs have been shown to reduce the incidence of human lung, colon, and breast cancer as well as rodent model tumors.43,48 They also seem to have therapeutic potential by targeting several independent pro-oncogenic mechanisms (eg, angiogenesis, transformation, and immunomodulation).49,50 PGE2 in particular induces anti-inflammatory reactions in macrophages, DCs, and T cells.51 The addition of PGE2 to in vitro-generated DCs during maturation seems to induce primarily a Th2-inducing phenotype, due to marked down-regulation of IL12.9,11,52-55 On the other hand, PGE2 seems to enhance antigen presentation and migration.11,54-56 Thus, the integrated effects of PGE2 on DC function and the impact of PGE2-maturated DCs on T-cell functions, especially in a tumor environment, remain to be established. Here, we have addressed the regulation of the 2 genes, namely INDO and IL2RA (CD25), both of which are most significantly induced by PGE2 in maturing DCs, in rendering these DCs immunoinhibitory. Immunohistochemistry of tumor specimens revealed that the corresponding proteins are regulated in vivo by DCs residing in close proximity of tumor cells.
Materials and methods
For further detail on methods, please refer to Document S1 (available at the Blood website; see the Supplemental Materials link at the top of the online article). Approval for these studies was obtained from the University of Cologne Institutional Review Board (Cologne, Germany).
In vitro generation of monocyte-derived DCs and enrichment of circulating DCs from peripheral blood
Positive enrichment of circulating monocytes and BDCA-1+ myeloid DCs was performed with CD14 MicroBeads and with CD1c (BDCA-1) Dendritic Cell Isolation Kit according to the manufacturer's protocols (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocyte-derived DCs were generated from CD14+ monocytes in a serum-free medium (CellGro DC medium; Cell Genix, Freiburg, Germany) with IL4 and granulocytemacrophage-colony-stimulating factor (GM-CSF) as previously described.57 Activation of DCs was performed in the presence of TNFα (20 ng/mL), combination of TNFα with αCD40 mAb (10 μg/mL), or a cytokine cocktail containing TNFα, IL6 (1000 U/mL), and IL1β (10 ng/mL) with or without PGE2 (1 μg/mL, equivalent to 2.84 μM). All adherent and nonadherent DCs were harvested and used together for the analyses described in “Results.” The phenotype of the in vitro-generated DCs was examined by flow cytometry using standard mAbs. For further detail on exact DC culture conditions, please refer to Document S1 and Figure S1. To assess the morphology of DCs, photographs of cells in 6-well Nunclon culture plates (Nunc, Karlsruhe, Germany) were taken by an Olympus C-2040 digital camera (Olympus, Tokyo, Japan) attached to an invertedphase Zeiss Telaval 31 microscope equipped with a 20 ×/0.35 Achromat CDN Ph objective (Zeiss, Oberkochen, Germany); an additional ×3 optical magnification via the camera was used.
RNA preparation, microarray hybridization, and microarray data processing
Preparation of cells and array hybridization were performed on a HG-U133A microarray platform (Affymetrix, Santa Clara, CA) as described previously.58 Data files were further analyzed with DNA-Chip Analyzer (dChip 1.2; www.dchip.org). Semiquantitative reverse-transcriptasepolymerase chain reaction (RT-PCR) was performed with a Qiagen One-Step RT-PCR kit (Qiagen, Valencia, CA) according to the manufacturer's recommendation.
Assessment of cytokines and tryptophan concentration in the supernatants
Production of IL12 (p70), soluble CD25 (sCD25), and IL2 was measured by corresponding Eli-Pair enzyme-linked immunosorbent assay (ELISA) kits (Diaclone Research, Besancon, France). The concentration of TNFα and IFNγ was measured using the human Th1/Th2 Cytokine kit II (BD Pharmingen, Heidelberg, Germany) according to the manufacturer's instructions. Measurement of tryptophan concentration was performed by reversedphase high-pressure liquid chromatography on a System Gold HPLC Workstation (Beckman, Munich, Germany) as previously described.32
Immunoblotting
Protein extracts were prepared from homogenized DCs, and protein concentration in samples was determined with Roti-Quant reagent (Carl Roth, Karlsruhe, Germany). SDS separation and staining with Coomassie Blue to assess the quality and loading were performed. Immunoblotting was performed as described previously59 using polyclonal (Serotec, Oxford, United Kingdom) and monoclonal (Chemicon, Hofheim, Germany) antibodies against IDO.
Functional assays with supernatants derived from monocyte-derived DCs
T-cell proliferation was determined after 4 days of stimulation with artificial antigen-presenting cells (aAPCs)60 at a ratio of 1:3 in the presence of supernatants from differentially cultured DCs. Exact assay conditions are described in Document S1. For mixed lymphocyte reaction, allogeneic CD4+ T cells (2 × 105 cells/well) were incubated for 4 to 6 days with differentially matured DCs in the presence or absence of corresponding supernatants at different ratios. Proliferation of T cells was assessed by CFSE staining according to the manufacturer's protocol (Molecular Probes, Eugene, OR).
Culture and assessment of cell proliferation and viability of CTLL-2 cells
The murine IL2-dependent T-cell line CTLL-2 was obtained from ATCC (TIB-214; Manassas, VA) and cultured in RPMI 1640 medium (Invitrogen, Karlsruhe, Germany) containing 0.1 mg/mL streptomycin, 100 U/mL penicillin (PAA Laboratories, Linz, Austria), 10% fetal calf serum (FCS; Invitrogen), and recombinant human IL2 (Chiron, Emeryville, CA). When used in functional assays, CTLL-2 cells were washed extensively and then cultured in supernatants derived from the indicated DCs. Supernatants were diluted with CTTL-2 culture medium at a 1:1 ratio. Cell proliferation was determined by cell counting (performed by 2 independent investigators), and viability assessed by flow cytometry measuring propidium iodidepositive cells.
Mixed lymphocyte reaction
Allogeneic CD4+ T cells (2 × 105 cells/well) were incubated for 4 to 6 days with differentially matured DCs in the presence or absence of corresponding supernatants at ratios of 1:1 to 1:100. T-cell proliferation was assessed by CFSE staining.
Immunohistochemical analysis
Immunohistochemical analysis of paraffin-embedded tissue samples of breast, colon, and gastric cancer (5 samples, each derived from independent patients) was performed using standard diagnostic protocols. Antibodies against S100 (DakoCytomation, Glostrup, Denmark), IDO (Serotec), or CD25 (Novocastra, Newcastle, United Kingdom) were used. Diagnosis was assessed by an experienced pathologist. Photographs were taken by a JVC KY-F75 U digital camera (JVC Germany, Friedburg, Germany) attached to a Zeiss Axiophot microscope. Zeiss 518 C immersion oil was used as imaging medium. For image acquisition, Diskus 4.60.343 software (Diskus, Koenigswinter, Germany) was used. For double staining, incubation with the first primary antibody (60 minutes) was followed by rat anti-mouse immunoglobulins (30 minutes), APAAP complex (30 minutes; DakoCytomation) and Vector red substrate kit (10 minutes; Vector, Burlingame, CA). Second primary antibody (60 minutes) was recognized by biotinylated secondary antibody (30 minutes), streptavidin-AP conjugate (30 minutes; DakoCytomation) and Vector Blue substrate kit (10 minutes; Vector).
Statistical analysis
Comparison between paired or unpaired groups was performed using the appropriate Student t test. A P value less than .05 was defined as statistically significant. All statistical analyses were performed using the SPSS statistical software package (SPSS 12.0 for Windows; SPSS, Chicago, IL).
Results
PGE2 changes morphology and reduces IL12 during DC maturation
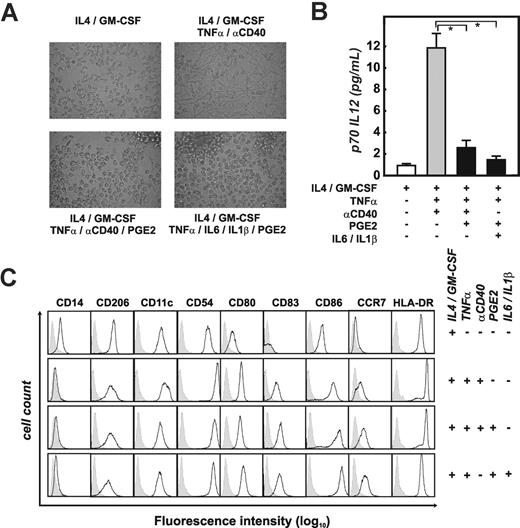
We primarily used human monocyte-derived immature DCs (imDCs) generated by culture of monocytes with GM-CSF and IL457 to study the role of PGE2 on DCs. Assessment of DC morphology, IL12 production, and phenotype was used to control the experimental system (Figure 1). While immature DCs (Figure 1A; GM-CSF/IL4) were loosely adherent, mostly of round shape with only short dendrites, maturation of DCs by TNFα and CD40 mAb (αCD40) induced many cells with long dendrites within 72 hours (Figure 1A, GM-CSF/IL4/TNFα/αCD40). DCs matured in the presence of PGE2 (matured by TNFα plus αCD40 or TNFα, IL6, and IL1β) were of round shape and showed only short cellular protrusions. These cells were mainly nonadherent and formed clusters within the culture (Figure 1A). Assessment of IL12 production demonstrated that PGE2 suppressed IL12 induced by DC maturation, independent of the maturation signals provided to imDCs (Figure 1B). An extended phenotypic analysis of imDC and all maDC cultures further demonstrated that expression of CD206, CD54, CD80, and HLA-DR was regulated and expressed during maturation independent of the presence of PGE2 (Figure 1C). Consistent with previously published data,54,56,61 expression of CD83, CD86, and CCR7 was slightly higher on DCs matured in the presence of PGE2. Overall, with these experiments, we established the conditions to assess the role of PGE2 during DC maturation on a genome-wide level.
Addition of PGE2 to maturing DCs changes their morphology and IL12 production. (A) Morphology of immature DCs (imDCs; IL4/GM-CSF) harvested at day 7, and mature monocyte-derived DC (maDCs) harvested at day 10. Different cytokine combinations were used during the last 72 hours of DC culture: DCs were matured with TNFα and αCD40 (n = 12) or with TNFα and αCD40 in the presence of PGE2 (n = 12). Alternatively, DC maturation was induced by TNFα, IL1β, and IL6 in the presence of PGE2 (n = 3). Photographs were taken by an Olympus digital camera (Tokyo, Japan) connected to a Zeiss Telaval 31 microscope. Representative experiments for each of the culture conditions are shown. (B) IL12 secretion measured in supernatants from different DC populations. Supernatants from imDCs (IL4 + GM-CSF) and maDCs (matured as described in panel A) were harvested 72 hours after the onset of maturation and assessed for p70 IL12 by ELISA, in at least 3 experiments per condition. Black bars represent IL12 production in PGE2-treated DCs; mean ± standard deviation is shown. *P < .05. (C) Analysis of cell-surface expression of CD14, CD206, CD11c, CD54, CD80, CD83, CD86, CCR7, and HLA-DR by different DC populations using flow cytometry. At least 12 experiments were performed for each of the conditions, except for the combination of TNFα, IL1β, IL6, and PGE2 (n = 3); a representative experiment for each condition is shown. Isotype controls are shown as gray area underneath the dotted line; specific antibodies are reflected by the black lines.
Addition of PGE2 to maturing DCs changes their morphology and IL12 production. (A) Morphology of immature DCs (imDCs; IL4/GM-CSF) harvested at day 7, and mature monocyte-derived DC (maDCs) harvested at day 10. Different cytokine combinations were used during the last 72 hours of DC culture: DCs were matured with TNFα and αCD40 (n = 12) or with TNFα and αCD40 in the presence of PGE2 (n = 12). Alternatively, DC maturation was induced by TNFα, IL1β, and IL6 in the presence of PGE2 (n = 3). Photographs were taken by an Olympus digital camera (Tokyo, Japan) connected to a Zeiss Telaval 31 microscope. Representative experiments for each of the culture conditions are shown. (B) IL12 secretion measured in supernatants from different DC populations. Supernatants from imDCs (IL4 + GM-CSF) and maDCs (matured as described in panel A) were harvested 72 hours after the onset of maturation and assessed for p70 IL12 by ELISA, in at least 3 experiments per condition. Black bars represent IL12 production in PGE2-treated DCs; mean ± standard deviation is shown. *P < .05. (C) Analysis of cell-surface expression of CD14, CD206, CD11c, CD54, CD80, CD83, CD86, CCR7, and HLA-DR by different DC populations using flow cytometry. At least 12 experiments were performed for each of the conditions, except for the combination of TNFα, IL1β, IL6, and PGE2 (n = 3); a representative experiment for each condition is shown. Isotype controls are shown as gray area underneath the dotted line; specific antibodies are reflected by the black lines.
Up-regulation of INDO and IL2RA by PGE2 in maturing DCs
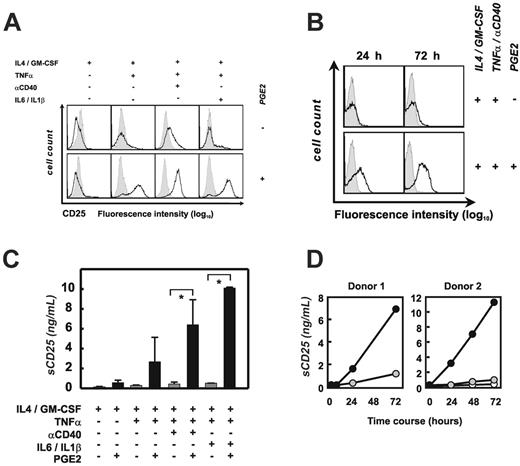
To better understand the overall impact of PGE2 stimulation in maDCs, we applied comprehensive gene expression profiling of maDCs in the presence (n = 3) or absence (n = 4) of PGE2 stimulation for 72 hours using the Affymetrix technology. Only 5 probe sets representing 4 genes passed our stringent filtering criteria for genes significantly up-regulated (Table S1). The number of genes down-regulated by PGE2 was also rather small (13 probe sets representing 12 genes). Among the up-regulated genes, we unexpectedly found indoleamine-pyrole 2,3 dioxygenase (INDO) to be the most strongly induced gene after PGE2 stimulation followed by IL2RA (the alpha chain of the IL2 receptor, CD25). Using real-time PCR, we demonstrated that both genes were induced as early as 6 hours after stimulation by PGE2 during DC maturation and were further up-regulated up to 72 hours (data not shown). The concomitant up-regulation of these 2 molecules was rather surprising since IDO has been associated with induction of tolerogenic T cells,21 while IL2RA induction on T cells was shown to be repressed by PGE2.62
Induction of CD25 in monocyte-derived DCs. (A) Analysis of cell-surface expression of CD25 by the different DC populations using flow cytometry. Three to 12 experiments were performed for each of the conditions; a representative experiment for each condition is shown. Isotype controls are shown as gray area underneath the dotted line; specific antibodies are reflected by the black lines. (B) Kinetics of surface CD25 expression on mo-DCs, matured with and without PGE2. Isotype controls are shown as gray area underneath the dotted line; specific antibodies are reflected by the black lines. One representative experiment of 2 is shown. (C) Soluble CD25 was assessed by ELISA in cell culture supernatants from different DC populations. Three to 12 experiments were performed for each of the conditions. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. (D) Kinetics of sCD25 production by mature mo-DCs under the influence of PGE2, as shown for 2 independent donors. ○ represents immature DCs; • DCs matured with TNFα and αCD40; and  , DCs matured with TNFα and αCD40 in the presence of PGE2.
, DCs matured with TNFα and αCD40 in the presence of PGE2.
Induction of CD25 in monocyte-derived DCs. (A) Analysis of cell-surface expression of CD25 by the different DC populations using flow cytometry. Three to 12 experiments were performed for each of the conditions; a representative experiment for each condition is shown. Isotype controls are shown as gray area underneath the dotted line; specific antibodies are reflected by the black lines. (B) Kinetics of surface CD25 expression on mo-DCs, matured with and without PGE2. Isotype controls are shown as gray area underneath the dotted line; specific antibodies are reflected by the black lines. One representative experiment of 2 is shown. (C) Soluble CD25 was assessed by ELISA in cell culture supernatants from different DC populations. Three to 12 experiments were performed for each of the conditions. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. (D) Kinetics of sCD25 production by mature mo-DCs under the influence of PGE2, as shown for 2 independent donors. ○ represents immature DCs; • DCs matured with TNFα and αCD40; and  , DCs matured with TNFα and αCD40 in the presence of PGE2.
, DCs matured with TNFα and αCD40 in the presence of PGE2.
CD25 protein is regulated by PGE2 on maturing DCs
Using flow cytometry we next assessed whether CD25 was also induced at the protein level. ImDCs were CD25-, and addition of PGE2 without maturation stimuli did not induce CD25 expression on the surface (Figure 2A, left). Accordingly, stimulation of imDCs with TNFα alone had no effect on CD25 expression. However, within 72 hours the combination of TNFα and PGE2 induced the majority of imDCs to express CD25 (Figure 2A, middle left). When maturing imDCs with TNFα and αCD40, low-level expression of CD25 was observed, while addition of PGE2 drastically increased the CD25 expression on the surface of the cells (Figure 2A, middle right). This was also true for DCs matured by a combination of TNFα with IL1β and IL6 in the presence of PGE2 (Figure 2A, right). Analysis of the kinetics of CD25 expression revealed that the protein is expressed on the DC surface within the first 24 hours and is further up-regulated throughout PGE2-driven maturation (Figure 2B). Taken together, these data demonstrate that CD25 expression on DCs is regulated by the interaction of TNFα and PGE2 and that other inflammatory signals do not further influence CD25 cell-surface expression.
Soluble CD25 (sCD25) is naturally released by proteolytic shedding from activated T cells, thereby representing a downregulatory mechanism of lymphocyte activation.63,64 sCD25 can reduce the level of cell-surface CD25 and can act as a soluble antagonist of IL2 action.65-67 We therefore measured sCD25 by ELISA in supernatants from DC cultures (Figure 2C). Independent of stimulation with PGE2, sCD25 was not detectable in supernatants from imDCs. Similar to cell-surface expression, TNFα and PGE2 were sufficient to induce sCD25, while TNFα alone did not induce sCD25. Accordingly, maturation with TNFα and αCD40 did not induce sCD25; however, when PGE2 was added, substantial amounts of sCD25 were detected in DC supernatants (Figure 2C). This was similarly true for the combination of TNFα, IL1β, IL6, and PGE2 (Figure 2C). In 2 donors, we measured the kinetics of sCD25 in DC supernatants. While sCD25 was not detectable in DC supernatants after 6 hours of culture, between 1.5 and 3 ng/mL sCD25 was detected only when DCs were matured in the presence of PGE2. The amount of sCD25 produced by PGE2-treated DCs increased until 72 hours of culture (Figure 2D).
CD25 is regulated by PGE2 on primary BDCA-1+ myeloid DCs
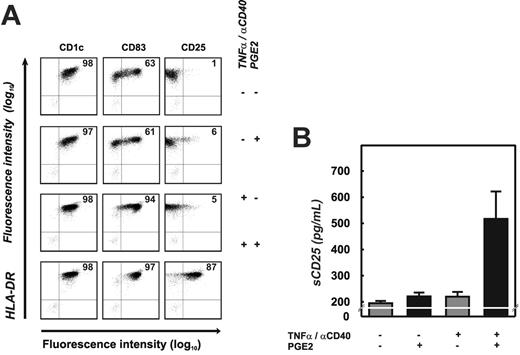
To determine if induction of CD25 was a function of monocytederived DCs or also of other myeloid DCs, we purified human BDCA-1+ myeloid DCs from peripheral blood. BDCA-1+ DCs were either cultured for 18 hours in medium alone or matured with αCD40 and TNFα in the presence or absence of PGE2. BDCA-1+ DCs cultured in medium partially matured in response to stimuli most likely resulting from adherence to plastic68 (and A.P., unpublished results, October 2004). This is reflected by upregulation of CD83 on a subpopulation of cells (Figure 3A, top panel). In the absence of PGE2, no CD25 surface expression was detected on these DCs (Figure 3A, top right dot plot). Addition of PGE2 did not alter the expression of CD83 on these cells, but low-level CD25 was detectable on the surface of a small percentage of cells (6%). When BDCA-1+ DCs were stimulated with αCD40 and TNFα, the majority of cells up-regulated CD83, but CD25 expression was seen only on a few cells (5%) at low expression levels. In contrast, maturation with αCD40 and TNFα in the presence of PGE2 resulted in up-regulation of CD83 on virtually all cells, and the great majority of these cells (87%) expressed high levels of CD25 on their surface.
Induction of CD25 in BDCA-1+ myeloid DCs. (A) Flow cytometry was performed after 18 hours of culture to assess cell-surface expression of CD1c, CD83, CD25 (x-axis), and HLA-DR (y-axis). At least 3 experiments were performed for each of the conditions; one representative experiment is shown. Percentage of events within the top right quadrant is stated. Greater than 99% of events in controls (cells labeled with isotype control antibodies) were within the bottom left quadrant. (B) Soluble CD25 was assessed by ELISA in cell culture supernatants from BDCA-1+ DCs after 18 hours. Black bars represent PGE2-treated DCs; mean ± standard deviation of 2 independent experiments is shown. Background sCD25 level in these experiments was 200 pg/mL.
Induction of CD25 in BDCA-1+ myeloid DCs. (A) Flow cytometry was performed after 18 hours of culture to assess cell-surface expression of CD1c, CD83, CD25 (x-axis), and HLA-DR (y-axis). At least 3 experiments were performed for each of the conditions; one representative experiment is shown. Percentage of events within the top right quadrant is stated. Greater than 99% of events in controls (cells labeled with isotype control antibodies) were within the bottom left quadrant. (B) Soluble CD25 was assessed by ELISA in cell culture supernatants from BDCA-1+ DCs after 18 hours. Black bars represent PGE2-treated DCs; mean ± standard deviation of 2 independent experiments is shown. Background sCD25 level in these experiments was 200 pg/mL.
When assessing sCD25 in supernatants of BDCA-1+ DCs, only cells activated with αCD40 and TNFα in the presence of PGE2 produced measurable amounts of this protein (Figure 3B). The lower amounts determined in cultures of BDCA-1+ DCs in comparison with the monocyte-derived DCs are likely due to the lower cell density and significantly shorter culture period (18 hours versus 24-72 hours, respectively). Taken together, up-regulation and consequent shedding of CD25 is a function of BDCA-1+ myeloid DCs as well as monocyte-derived DCs, and is dependent on an integration of maturation signals and PGE2-mediated signaling.
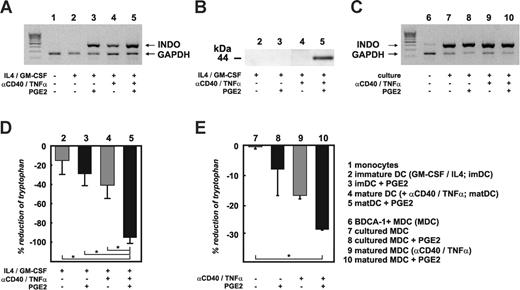
Expression and function of IDO in monocyte-derived DCs and myeloid BDCA-1+ DCs. mRNA for RT-PCR and cell lysates for immunoblot analysis were obtained from monocytes, imDCs, or maDCs cultured in the presence or absence of PGE2. Similarly, BDCA-1+ myeloid DCs were cultured for 18 hours in the presence or absence of PGE2, αCD40, and TNFα, combined as indicated. (A) Identification of IDO regulation on mRNA level by RT-PCR using forward and reverse IDO primers spanning exons 6 to 10. Two additional primer sets spanning exons 1 to 7 and 8 to 10 of IDO were also used and gave the same results. Results shown are representative of at least 3 independent experiments. (B) IDO protein expression in cell lysates from different DCs was determined by at least 5 independent immunoblot analyses using a monoclonal or polyclonal IDO antibody. Shown here are data using the monoclonal antibody. (C) IDO RNA was also assessed in BDCA-1+ DCs upon purification and after short-term in vitro culture using the same primer sets. (D) Enzymatic activity of IDO was determined measuring tryptophan levels in supernatants derived from imDCs or maDCs in the presence or absence of PGE2 by reversed-phase HPLC analysis. Except for imDCs stimulated with PGE2 alone (n = 2), at least 10 experiments were performed for the other conditions. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. (E) IDO activity as assessed by tryptophan reduction in supernatants of short-term cultured BDCA-1+ DCs under the same culture conditions as described for Figure 4C (n = 2). Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05.
Expression and function of IDO in monocyte-derived DCs and myeloid BDCA-1+ DCs. mRNA for RT-PCR and cell lysates for immunoblot analysis were obtained from monocytes, imDCs, or maDCs cultured in the presence or absence of PGE2. Similarly, BDCA-1+ myeloid DCs were cultured for 18 hours in the presence or absence of PGE2, αCD40, and TNFα, combined as indicated. (A) Identification of IDO regulation on mRNA level by RT-PCR using forward and reverse IDO primers spanning exons 6 to 10. Two additional primer sets spanning exons 1 to 7 and 8 to 10 of IDO were also used and gave the same results. Results shown are representative of at least 3 independent experiments. (B) IDO protein expression in cell lysates from different DCs was determined by at least 5 independent immunoblot analyses using a monoclonal or polyclonal IDO antibody. Shown here are data using the monoclonal antibody. (C) IDO RNA was also assessed in BDCA-1+ DCs upon purification and after short-term in vitro culture using the same primer sets. (D) Enzymatic activity of IDO was determined measuring tryptophan levels in supernatants derived from imDCs or maDCs in the presence or absence of PGE2 by reversed-phase HPLC analysis. Except for imDCs stimulated with PGE2 alone (n = 2), at least 10 experiments were performed for the other conditions. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. (E) IDO activity as assessed by tryptophan reduction in supernatants of short-term cultured BDCA-1+ DCs under the same culture conditions as described for Figure 4C (n = 2). Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05.
Induction of functional IDO in monocyte-derived DCs by PGE2
We next verified INDO mRNA regulation by RT-PCR using 3 independent primer sets spanning all exons of INDO. Identical to microarray analysis, INDO mRNA was absent in monocytes or imDCs (Figure 4A). Up-regulation of INDO mRNA by imDCs was induced by either PGE2 alone, maturation with TNFα and αCD40, or the combination of all 3 factors (Figure 4A) or when DCs were matured by TNFα, IL1β, IL6, and PGE2 (data not shown). Semiquantitative analysis revealed highest mRNA levels for IDO in DCs treated with PGE2, again corroborating the microarray analysis (data not shown). These results were similar for all RT-PCRs performed with the 3 different primer sets. No evidence was found for induction of splice variants by the different stimuli (Figure 4A and data not shown). Using a monoclonal antibody, we assessed IDO protein expression in cell lysates from different DCs (Figure 4B). ImDCs showed absence of IDO protein, similar to mRNA. In contrast, while INDO mRNA was clearly induced in imDCs in the presence of PGE2, this did not result in detectable protein expression, suggesting that additional signals are necessary to induce translation of INDO mRNA. Similarly, although maturation of DCs in the presence of TNFα and αCD40 induced INDO mRNA, it did not lead to IDO protein expression. However, when assessing IDO protein in maDCs under the influence of PGE2, strong induction of IDO protein expression was detected (Figure 4B). Similar data were obtained with a polyclonal IDO-specific antibody (data not shown).
Since IDO apparently is regulated on several levels within human DCs,19 we next assessed whether IDO is functional in DCs expressing IDO protein (Figure 4D). Following previously described methods,32 we analyzed tryptophan concentration in culture supernatants as a function of IDO enzymatic activity. As expected, in the absence of IDO protein expression, tryptophan levels were basically unchanged in cultures from imDCs. The addition of PGE2 to imDCs led to a small but not statistically significant decrease of tryptophan when compared with imDCs in the absence of PGE2. Similarly, tryptophan levels were also slightly reduced in maDCs, suggesting that these cells may exhibit some low-level enzymatic activity of IDO that was undetectable by immunoblotting. In contrast, in supernatants derived from DCs matured in the presence of PGE2, tryptophan was almost completely ablated, which is indicative of a significant increase in enzymatic activity of IDO and correlates with the induction of IDO protein (Figure 4B). Significant reduction of tryptophan concentration in DC supernatants was also noticed when DCs were matured in the presence of TNFα and PGE2, or with a combination of TNFα, IL6, IL1β, and PGE2 (data not shown). Taken together, these data support a complex transcriptional and posttranscriptional regulation of IDO in human DCs. Maturation signals or PGE2 alone is sufficient to induce mRNA, while only the integration of maturation and PGE2 signals induces IDO protein expression with full enzymatic activity.
Maturation signals and PGE2 are also required to induce functional IDO in BDCA-1+ myeloid DCs
We performed RT-PCR to detect INDO mRNA in BDCA-1+ myeloid DCs (Figure 4C). Since BDCA-1+ DCs are at least partially maturing solely in response to adherence on plastic (Figure 3A and A.P., unpublished results, October 2004), we postulated that INDO mRNA might already be induced under these conditions. Indeed, while INDO mRNA was present at very low levels in highly purified BDCA-1+ DCs (purity > 98%) prior to culture, it was significantly up-regulated 18 hours after culture on plastic. Under these conditions, no further up-regulation of INDO mRNA by PGE2, TNFα, and αCD40 was observed (Figure 4C).
Since cell numbers of BDCA-1+ cells from peripheral blood were regularly too low to perform immunoblot analysis, we assessed only tryptophan catabolism in supernatants of short-term cultured BDCA-1+ DCs (18 hours, Figure 4E). Without stimulation by TNFα and αCD40 or PGE2, no tryptophan depletion was observed. PGE2 alone or the combination of TNFα and αCD40 induced some tryptophan depletion. However, again, the combination of DC activation by TNFα and αCD40 in the presence of PGE2 signaling led to the highest depletion of tryptophan in these short-term cultures. Reduction of tryptophan in BDCA-1+ DC cultures was lower than observed for monocyte-derived DCs. However, this is most likely due to the shorter incubation time, as well as lower cell density in BDCA-1+ DC cultures. These data further support that induction of functional IDO by PGE2 is not restricted to in vitro-generated monocyte-derived DCs but also applies to primary BDCA-1+ myeloid DCs.
Monocyte-derived DCs retain the expression of functional IDO and CD25 after removal of PGE2 and maturation signals. (A) IDO protein expression and enzymatic activity as measured by tryptophan levels 24 hours after washout (6 independent experiments). Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. (B) Surface expression of CD25 on mature DCs before and 24 hours after washout. One representative experiment of 4 is shown. (C) Production of soluble CD25 as determined by ELISA from supernatants after washout. Shown here is the mean ± SD of 6 independent experiments. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05.
Monocyte-derived DCs retain the expression of functional IDO and CD25 after removal of PGE2 and maturation signals. (A) IDO protein expression and enzymatic activity as measured by tryptophan levels 24 hours after washout (6 independent experiments). Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. (B) Surface expression of CD25 on mature DCs before and 24 hours after washout. One representative experiment of 4 is shown. (C) Production of soluble CD25 as determined by ELISA from supernatants after washout. Shown here is the mean ± SD of 6 independent experiments. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05.
Soluble factors secreted by PGE2-treated matured DCs inhibit T-cell activation
To address whether the induction of IDO and sCD25 would exert functional consequences on T cells, we first tested the effect of DC supernatants on T-cell activation. Since PGE2 is known to suppress T-cell function directly, PGE2 was removed from the cultures by extensively washing (“washout”) the DC prior to further culture (Figure S1). Fresh DC medium lacking the exogenous stimuli (TNFα, αCD40, PGE2) was then added and DCs were incubated for an additional 24 hours prior to collection of cells and supernatants. As shown in Figure 5A, DCs matured in the presence of PGE2 retained IDO expression even 24 hours after removal of PGE2 and all maturation signals. Surprisingly, a weak band at 44 kDa was also observed in cell lysates derived from DCs matured in the absence of PGE2. Whether the induction of low-level IDO under these conditions is due to up-regulation of endogenously expressed PGE2 by mature DCs cannot be completely ruled out in these experiments. IDO in PGE2-treated DCs displayed still a strong enzymatic activity 24 hours after washout as demonstrated by significantly reduced levels of tryptophan in supernatants from these cells (Figure 5A). When assessing CD25 protein, PGE2-stimulated DCs still expressed cell surface CD25 (Figure 5B, bottom right panel) and sCD25 (Figure 5C) 24 hours after washout. Despite a shortened culture period (24 hours), the concentration of sCD25 after washout was comparable with the levels observed in supernatants from maturing DCs (Figure 2).
As a function of T-cell activation, we first measured proliferation, and the TNFα and IFNγ production of purified human CFSE-labeled CD4+ T cells stimulated with artificial APCs,60 in the presence of supernatants derived from the different DC populations after washout. Between 47% and 56% of CD4+ T cells proliferated in the presence of supernatants derived from DCs matured in the absence of PGE2 (Figure 6A, left column). This is identical to results obtained from CD4+ T cells cultured in fresh CellGro medium (data not shown). In contrast, when T-cell stimulation was performed in supernatants collected from PGE2-treated DCs after washout, a significant inhibitory effect on proliferation was observed (Figure 6A, middle column). Similarly, the secretion of IFNγ and TNFα by CD4+ T cells was reduced in the presence of these supernatants (data not shown).
To evaluate the role of tryptophan levels on proliferation and cytokine production, tryptophan concentrations in supernatants derived after washout from PGE2-treated DCs were adjusted to concentrations measured in supernatants from corresponding maDCs (Figure 6A, right column). When replenishing tryptophan, we observed 2 principle patterns. In the first group, restoring tryptophan levels in the supernatant prior to use in T-cell activation assays resulted in a significant although not complete recovery of T-cell proliferation (Figure 6A, top row) as well as cytokine production (data not shown). In other donors (Figure 6A, bottom row), tryptophan replacement had no effect, indicating that factors other than tryptophan depletion might be involved in inhibiting T-cell activation. Of interest, these DC supernatants were characterized by a significantly higher concentration of sCD25 (Figure 6A, right).
To assess whether CD25 expressed and secreted by DCs matured in the presence of PGE2 can bind IL2 and thereby sequester this cytokine, we matured DCs in the absence or presence of PGE2 as described in “Materials and methods.” Twenty-four hours after the onset of maturation, half of the cultures were pulsed with recombinant IL2, and at day 10 of culture, the concentration of IL2 in these supernatants was assessed by ELISA. We observed a significant reduction (43% ± 4%, mean ± SD) of recombinant IL2 in supernatants from PGE2-treated DCs in comparison with DCs matured in absence of PGE2 (data not shown). No IL2 was detected in DC cultures where no recombinant IL2 was added before, demonstrating that DCs do not secrete IL2 under the chosen culture conditions. To further demonstrate the functional consequence of sequestration of IL2 by DCs expressing and secreting CD25, we measured viability and cell proliferation of IL2-dependent CTLL-2 cells after 48 hours of incubation with the respective DC supernatants. As shown in Figure 6B, in cultures of CTLL-2 cells exposed to supernatants from DCs without prior addition of recombinant IL2, more than 80% of cells had died and no cell proliferation occurred. Strong cell proliferation and high viability of CTLL-2 was observed when cultured in IL2-containing supernatants from DCs matured without PGE2, demonstrating that these cells did not sequester IL2 (Figure 6B). In contrast, when IL2-containing supernatants from PGE2-treated DCs were used, cell proliferation was significantly lower and viability reduced, further demonstrating that CD25-expressing and -secreting DCs sequester IL2, thereby reducing the function of IL2-dependent CTLL-2 cells (Figure 6B).
Next, we assessed if blockade of IDO by using the competitive inhibitor 1-methyltryptophan (1-MT) would have an outcome similar to tryptophan replacement (Figure 6A).20 We therefore incubated DCs matured in the presence of PGE2 with 1-MT for 24 hours after washout. As shown in Figure 6C, more than half (55%) of the CD4+ T cells stimulated by aAPCs in supernatants from maDCs proliferated, and 40% had divided more than once as assessed by CFSE staining (Figure 6C, top histogram). In contrast, only 42% of CD4+ T cells stimulated in supernatants of PGE2-treated matured DCs proliferated, but of more importance, only 22% had divided more than once (Figure 6C, middle histogram). This inhibitory effect was partially reversed by the addition of 1-MT to DCs. Similar to the control experiment (Figure 6C, upper histogram), more than half of all CD4+ T cells proliferated and 34% had divided at least twice (Figure 6C, lower histogram). These data further support the role of functional expression of IDO for the observed inhibition of T-cell proliferation (Figure 6C) and cytokine production (data not shown).
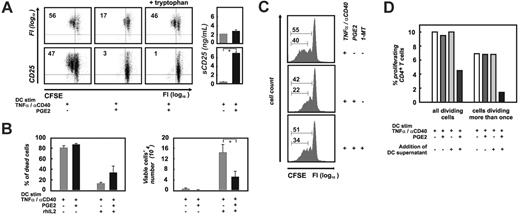
Influence of DC supernatants on T-cell proliferation. (A) Purified human CD4+ T cells were stimulated with aAPCs in the presence of supernatants derived 24 hours after washout from differentially treated DCs, or DC-related soluble factors (sCD25). Proliferation was assessed by flow cytometry using CFSE-labeled CD4+ T cells; CD25 costaining was used as a control of proliferation. In PGE2-treated samples, the tryptophan concentration was adjusted (+ tryptophan, right column of dot plots) to the corresponding concentration measured in the control supernatant (left column of dot plots). On the right, soluble CD25 concentrations for the corresponding DC supernatants are shown. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. Two representative experiments of 9 are shown. (B) Analysis of cell viability (left panel) and cell proliferation (right panel) of the IL2-dependent cell line CTLL-2. Supernatants from DCs matured with TNFα and αCD40 in the presence or absence of PGE2 were added to CTLL-2 cells. Where indicated, recombinant IL2 was added to DC cultures during the last 48 hours of maturation. Cell viability and cell numbers of CTLL-2 cells were assessed at 48 hours. CTLL-2 cultures exposed to PGE2-treated supernatants are represented by black bars. Mean ± standard deviation of at least 3 experiments is shown. *P < .05. (C) Proliferation of human T cells stimulated by aAPCs as described in panel A. Influence of blockade of IDO by 1-MT (1 mM) after washout during the last 24 hours of DC culture prior to collection of the supernatant (right histogram plot) was studied. One of 3 experiments is shown. (D) DC-derived soluble factors suppress the alloantigen-specific T-cell activation. Purified human CFSE-labeled allogeneic CD4+ T cells were incubated with DCs matured with or without PGE2 in the presence or absence of the corresponding DC supernatant or fresh medium. Shown here are the percentage of all dividing cells as well as the percentage of cells dividing more than once. At least 30 000 events within the positive gate were assessed for each of the conditions shown here. Reanalysis was performed at least twice for each experiment by 2 individual investigators and shown here is 1 representative experiment of 3. (White bars) Percentage of allogeneic T cells proliferating in response to maDCs in absence of PGE2; MLR was performed in fresh medium. (Dark gray bars) Percentage of allogeneic T cells proliferating in response to maDCs in presence of PGE2; MLR was performed in fresh medium. (Light gray bars) Percentage of allogeneic T cells proliferating in response to maDCs in absence of PGE2; MLR was performed in supernatants derived from maDCs. (Black bars) Percentage of allogeneic T cells proliferating in response to maDCs in presence of PGE2; MLR was performed in supernatants derived from maDCs in presence of PGE2.
Influence of DC supernatants on T-cell proliferation. (A) Purified human CD4+ T cells were stimulated with aAPCs in the presence of supernatants derived 24 hours after washout from differentially treated DCs, or DC-related soluble factors (sCD25). Proliferation was assessed by flow cytometry using CFSE-labeled CD4+ T cells; CD25 costaining was used as a control of proliferation. In PGE2-treated samples, the tryptophan concentration was adjusted (+ tryptophan, right column of dot plots) to the corresponding concentration measured in the control supernatant (left column of dot plots). On the right, soluble CD25 concentrations for the corresponding DC supernatants are shown. Black bars represent PGE2-treated DCs; mean ± standard deviation of all experiments is shown. *P < .05. Two representative experiments of 9 are shown. (B) Analysis of cell viability (left panel) and cell proliferation (right panel) of the IL2-dependent cell line CTLL-2. Supernatants from DCs matured with TNFα and αCD40 in the presence or absence of PGE2 were added to CTLL-2 cells. Where indicated, recombinant IL2 was added to DC cultures during the last 48 hours of maturation. Cell viability and cell numbers of CTLL-2 cells were assessed at 48 hours. CTLL-2 cultures exposed to PGE2-treated supernatants are represented by black bars. Mean ± standard deviation of at least 3 experiments is shown. *P < .05. (C) Proliferation of human T cells stimulated by aAPCs as described in panel A. Influence of blockade of IDO by 1-MT (1 mM) after washout during the last 24 hours of DC culture prior to collection of the supernatant (right histogram plot) was studied. One of 3 experiments is shown. (D) DC-derived soluble factors suppress the alloantigen-specific T-cell activation. Purified human CFSE-labeled allogeneic CD4+ T cells were incubated with DCs matured with or without PGE2 in the presence or absence of the corresponding DC supernatant or fresh medium. Shown here are the percentage of all dividing cells as well as the percentage of cells dividing more than once. At least 30 000 events within the positive gate were assessed for each of the conditions shown here. Reanalysis was performed at least twice for each experiment by 2 individual investigators and shown here is 1 representative experiment of 3. (White bars) Percentage of allogeneic T cells proliferating in response to maDCs in absence of PGE2; MLR was performed in fresh medium. (Dark gray bars) Percentage of allogeneic T cells proliferating in response to maDCs in presence of PGE2; MLR was performed in fresh medium. (Light gray bars) Percentage of allogeneic T cells proliferating in response to maDCs in absence of PGE2; MLR was performed in supernatants derived from maDCs. (Black bars) Percentage of allogeneic T cells proliferating in response to maDCs in presence of PGE2; MLR was performed in supernatants derived from maDCs in presence of PGE2.
To further study the role of soluble factors derived from PGE2-pretreated DCs, we assessed CFSE-labeled alloantigenspecific T-cell activation induced by the different DC populations in the presence or absence of the respective DC supernatants. Alloantigen was used as a model based on the high prevalence of up to 10% of CD4+ alloantigen-specific T cells in humans. When DCs were extensively washed prior to use in mixed lymphocyte reactions (MLRs, Figure 6D, white bars and dark gray bars), approximately 10% of all CD4+ T cells proliferated irrespective of treatment of DCs with PGE2. This is in line with previous findings demonstrating APC function of DCs matured even in the presence of PGE2.69 However, when MLRs were performed in the presence of the respective DC supernatants (after washout, Figure 6D, light gray and black bars), T-cell proliferation induced by PGE2-treated DCs was significantly reduced. T-cell inhibition was furthermore reflected by a significant reduction of IFNγ and TNFα secretion by the T cells (data not shown). In control experiments using DCs matured without PGE2 and their respective supernatants (Figure 6D, light gray bars), proliferation was unchanged.
When assessing cells that had divided more than once, the effect was even more pronounced. Only 1.4% of CD4+ T cells stimulated by PGE2-treated DCs in the presence of supernatants (after washout) had divided more than once, while in all other conditions more than 6.8% had divided at least twice. These data further support the inhibitory effect of soluble factors derived from DCs matured in the presence of PGE2.
Peritumoral dendritic cells coexpress CD25 and IDO
To assess the role of concomitant CD25 and IDO expression in the context of antitumor immune responses, 3 different carcinoma entities (mamma, n = 5; colon, n = 5; and gastric tumors, n = 5) with distinct peritumoral inflammatory reaction were chosen for immunohistochemical (IH) analysis. A representative gastric carcinoma sample is shown in Figure 7. In all carcinomas, S100+ DCs were detected in the proximity of the cancer cells (Figure 7B). In double-staining experiments, approximately one third of the peritumoral DCs presented coexpression of IDO, while additional IDO+ cells were located in close vicinity to these IDO+ DCs (Figure 7B).
In tumor-draining lymph nodes, we observed low numbers of S100+ DCs, of which approximately one third coexpressed IDO (Figure 7C). When CD25 was assessed in the context of DCs, we clearly observed a population of DCs coexpressing CD25; however, there were even more cells negative for S100 but expressing CD25. Of interest, these CD25+ cells were in close proximity to CD25+ S100+ DCs (Figure 7D). We also identified CD25+ S100+ DCs within the tumor masses (Figure 7E). These observations were very similar in all 3 tumor entities studied.
Discussion
Induction of tolerance to tumor-associated antigens is an immunologic hallmark during tumor development and progression. Tolerogenic DCs have been implicated to play an important role in this process. The expression of IDO in DCs has been linked to the induction of such a tolerogenic phenotype,28,35 and more recently, PGE2 was suggested to induce IDO in in vitro-generated DCs.38 However in other reports, PGE2 has also been shown to enhance antigen presentation and migration of DCs.11,54-56 Thus, the global consequences of PGE2-maturated DCs on T-cell functions needed to be further elucidated. Additionally, it is critical to demonstrate whether the regulation of IDO in DCs can also be observed in vivo in the context of malignant disease.
Expression of CD25 and IDO in peritumoral dendritic cells. Example of a gastric carcinoma with considerable inflammatory reaction. Photographs were taken by a JVC digital camera connected to a Zeiss Axiophot microscope. (A) Overview of a sample slide with invasive tumor cell populations and peritumoral inflammatory reaction (magnification, × 100; hematoxylin-eosin staining). (B) Magnification of the border area of the carcinoma: double-immunostaining for S100 (red) and IDO (blue) demonstrates a distinct population of purple-colored double-stained cells (#); magnification, × 1000. (C) Tumor-draining lymph node next to the carcinoma: double immunostaining for S100 (red) and IDO (blue). S100 single-positive DCs (red) as well as S100/IDO-coexpressing cells (purple colored, #) are seen; magnification × 1000. (D) Peritumoral CD25+ cells (red) are more numerous than S100+ DCs (blue). Few double-stained purple cells are visible (#); magnification, × 250. (E) Intratumoral S100+ DCs in close proximity to double-stained S100+ CD25+ DCs (purple colored, #); magnification, × 400. For panel A, a 10 ×/0.32 Plan Apochromat objective lens was used to visualize the image; for panels B-E, a 63 ×/1.40 oil-immersion Plan Apochromat objective lens was used to visualize images.
Expression of CD25 and IDO in peritumoral dendritic cells. Example of a gastric carcinoma with considerable inflammatory reaction. Photographs were taken by a JVC digital camera connected to a Zeiss Axiophot microscope. (A) Overview of a sample slide with invasive tumor cell populations and peritumoral inflammatory reaction (magnification, × 100; hematoxylin-eosin staining). (B) Magnification of the border area of the carcinoma: double-immunostaining for S100 (red) and IDO (blue) demonstrates a distinct population of purple-colored double-stained cells (#); magnification, × 1000. (C) Tumor-draining lymph node next to the carcinoma: double immunostaining for S100 (red) and IDO (blue). S100 single-positive DCs (red) as well as S100/IDO-coexpressing cells (purple colored, #) are seen; magnification × 1000. (D) Peritumoral CD25+ cells (red) are more numerous than S100+ DCs (blue). Few double-stained purple cells are visible (#); magnification, × 250. (E) Intratumoral S100+ DCs in close proximity to double-stained S100+ CD25+ DCs (purple colored, #); magnification, × 400. For panel A, a 10 ×/0.32 Plan Apochromat objective lens was used to visualize the image; for panels B-E, a 63 ×/1.40 oil-immersion Plan Apochromat objective lens was used to visualize images.
In this report, we extend previous findings and demonstrate that DCs matured in the presence of PGE2 induce not only IDO expression but also surface expression and secretion of CD25. The consequences on T-cell function are inhibitory, mainly mediated by tryptophan depletion; however, soluble CD25 also seems to act in an inhibitory manner on T-cell activation, as demonstrated by CTLL-2 assays. We further demonstrate coexpression of CD25 and IDO in DCs within the tumor microenvironment in different cancer entities, underscoring the impact of PGE2-mediated expression of both molecules in vivo in the context of malignant disease.
Demonstrating the concomitant induction of IDO enzymatic activity and sCD25 by matured DCs after PGE2 stimulation, we provide 2 independent mechanisms that have been associated with induction of T-cell tolerance and immune inhibition. IDO is subject to transcriptional and translational regulation by PGE2 in concert with maturation signals. While PGE2 up-regulates INDO mRNA, it is not sufficient to induce IDO protein in immature DCs. Maturation signals (eg, provided by TNFα and αCD40 stimulation) are also sufficient to induce INDO mRNA, but the expression of IDO protein requires integration of maturation signals and PGE2. Of importance, we demonstrate that the observed effect of PGE2 is not limited to in vitro-generated monocyte-derived DCs, the induction of functional IDO, expression of cell-surface CD25, and secretion of sCD25 are equally dependent upon the integration of maturation signals and PGE2 in primary BDCA-1+ myeloid DCs. Neither PGE2 nor maturation (eg, by TNFα and αCD40) alone induce functional IDO or CD25 in these primary cells.
Initially, CD25 has been introduced as a maturation marker on DCs; however, its function is still discussed controversially.70,71 Our observation of cell-surface expression of the activation marker CD25 on PGE2-treated matured DCs was surprising. CD25-expressing DCs did not produce IL2; moreover, stimulation of surface CD25 with exogenous IL2 revealed no effect on DC phenotype (A.P., unpublished results, May 2005). Even more surprising was the significant production of sCD25 by these cells. To this end, we clearly demonstrate that T-cell inhibition is due mainly to reduction of tryptophan by PGE2-pretreated DCs. Because replenishing tryptophan did not entirely restore T-cell proliferation, other factors such as CD25 expression and secretion by DCs might also be involved in T-cell inhibition. Indeed, we were able to demonstrate that DCs expressing and secreting CD25 can inhibit cell proliferation of the IL2-dependent CTLL-2 cell line used as readout for sequestration of IL2 by these DCs.
The important role of integrating soluble factors derived from DCs when analyzing their APC function was also demonstrated by allo-MLR. Clearly, DCs in the context of their supernatants showed inhibitory function on T-cell activation and proliferation, while DCs in the absence of their respective supernatants were stimulatory. The latter finding corresponds to previously published data demonstrating that DCs matured in the presence of TNFα, IL1β, IL6, and PGE256 show activating effects on T cells in the absence of soluble factors derived from DCs. In preliminary experiments, we have observed that the inhibitory effect of the DC supernatants on individual T-cell subsets (naive CCR7+CD45RA+, central memory CCR7+CD45RA-, or effector memory CCR7-CD45RA- T cells) seems to be dependent on the strength of the T-cell-receptor signal (M.B., unpublished results, July 2005). Naive, central, and effector memory T cells were similarly inhibited to proliferate upon stimulation by strong TCR signals (as provided by aAPCs) in the presence of supernatants derived from PGE-treated DCs. In contrast, when T cells were stimulated by allogeneic DCs, naive and central memory subsets were similarly inhibited to proliferate by addition of supernatants from PGE2-treated DCs, while the low percentage of proliferating effector memory T cells remained unchanged (M.B., unpublished results, July 2005). It will be of interest to study whether other immune effector cells such as CD8+ T cells, γδT cells, natural killer T (NKT) cells, or natural killer (NK) cells are also influenced by PGE2-stimulated DCs.
Using 3 different tumor entities, we have been able to demonstrate that DCs in the tumor microenvironment in fact express CD25 and IDO. These data are of notable interest, since elevated PGE2 concentrations in the tumor environment might substantially differ from PGE2 concentrations used in in vitro experiments. Given the fact that all analyzed tumor samples (mamma, colon, and gastric tumors) are described to be associated with increased PGE2 concentrations, we hypothesize that PGE2 induces tolerogenic DCs expressing CD25 and IDO in the environment of these tumors. By at least 2 distinct mechanisms, namely by depleting the concentration of tryptophan and by secretion of soluble CD25, these DCs act to impair helper T-cell function.
Taken together, we demonstrate that PGE2, an important promoter of tumorigenesis and immune evasion in cancer and tumor-induced T-cell tolerance, mediates the induction of enzymatic activity of IDO and soluble CD25 in DCs. Whether these PGE2-induced mechanisms are also used by other immune inhibitory factors released by tumors including IL10, TGFβ, or VEGF will be a major focus of future research. In light of the promising results from preclinical in vivo data successfully targeting IDO in combination with chemotherapy,25 it will be necessary to further dissect these inhibitory circuits as a prerequisite to combine molecularly defined immunotherapies and chemotherapies in cancer patients.
Prepublished online as Blood First Edition Paper, March 7, 2006; DOI 10.1182/blood-2005-08-3507.
Supported by a Sofja Kovalevskaja Award from the Alexander von Humboldt Foundation (J.L.S.), a Max-Eder Translational Research Award (M.S.v.B.-B.), a grant from the Deutsche Forschungsgemeinschaft (SFB635, F.-G.H.), and a Mildred Scheel Fellowship (J.C.) from the Deutsche Krebshilfe.
M.S.v.B.-B. and J.L.S. designed research, analyzed data, and wrote the paper; A.P. designed research, performed research, analyzed data, and wrote the paper; J.C. and C.W. performed research, analyzed data, and wrote the paper; S.C., M.S.S., F.F., and U.R. performed research; and T.S., M.B., S.D., and F.-G.H. performed research and analyzed data.
M.S.v.B.-B. and A.P. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank our colleagues from the Center for Transfusion Medicine for providing us with peripheral blood products. We are grateful to all blood donors for donating blood for this study. We are also indebted to Jim Riley (Abramson Cancer Research Center, University of Pennsylvania, Philadelphia) for providing the anti-CD28 mAb 9.3.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal