In mice, interleukin-7 (IL-7) and IL-15 are involved in T-cell homeostasis and the maintenance of immunologic memory. Here, we follow virus-induced responses in infectious mononucleosis (IM) patients from primary Epstein-Barr virus (EBV) infection into long-term virus carriage, monitoring IL-7 and IL-15 receptor (IL-R) expression by antibody staining and cytokine responsiveness by STAT5 phosphorylation and in vitro proliferation. Expression of IL-7Rα was lost from all CD8+ T cells, including EBV epitope-specific populations, during acute IM. Thereafter, expression recovered quickly on total CD8+ cells but slowly and incompletely on EBV-specific memory cells. Expression of IL-15Rα was also lost in acute IM and remained undetectable thereafter not just on EBV-specific CD8+ populations but on the whole peripheral T- and natural killer (NK)-cell pool. This deficit, correlating with defective IL-15 responsiveness in vitro, was consistently observed in patients up to 14 years after IM but not in patients after cytomegalovirus (CMV)-associated mononucleosis, or in healthy EBV carriers with no history of IM, or in EBV-naive individuals. By permanently scarring the immune system, symptomatic primary EBV infection provides a unique cohort of patients through which to study the effects of impaired IL-15 signaling on human lymphocyte functions in vitro and in vivo. (Blood. 2006;108:11-18)
Introduction
The immune system relies on tightly controlled mechanisms to regulate the size of lymphocyte pools. From work in mouse systems, cytokines of the common γ chain (γc) family appear to play an important role in T-cell homeostasis.1 In particular, interleukin-7 (IL-7) is required for the maintenance and homeostatic proliferation of both naive and memory T cells2,3 and is thought to act principally through up-regulation of antiapoptotic proteins. In parallel, IL-15 is involved in the generation and homeostatic (antigen-independent) maintenance of memory CD8+ T cells through acting as a growth, and possibly also as an antiapoptotic, factor.4-6 T-cell responsiveness to these cytokines is in each case determined by expression of the relevant receptor on the cell surface. The IL-7 receptor is a heterodimer consisting of an α chain (CD127) that binds IL-7 with high-affinity (Ka = 10-10 M) and the common γc (CD132). By contrast, the IL-15 receptor involves a private α chain, a β subunit (CD122) shared with the IL-2 receptor, and the common γc. The α chain alone binds to IL-15 with high affinity (Ka = 10-11 M), and in this case the β/γ complex alone can also bind the cytokine, albeit with much lower affinity.7 Both the IL-7 and IL-15 receptors signal via Janus kinase (JAK) family members; in both cases, the α chain signals via JAK1 and the γc via JAK3, leading to the nuclear translocation of STAT3 and STAT5, respectively.8
Of particular interest is the role that these cytokines may also play in the regulation of antigen-dependent responses. In mouse models, this is best studied in the context of CD8+ T-cell responses induced by viral infection. Thus, IL-15-deficient mice show reductions in the initial expansion of effector CD8+ T cells that typify acute viral infection,9,10 implying a role for this cytokine in the generation and proliferation of reactive cells. IL-15Rα expression is indeed reported to be enhanced on recently activated mouse CD8+ T cells.4,10 By contrast, expression of the IL-7Rα is down-regulated on most if not all activated cells during acute primary infection.11,12 Of interest, however, this receptor is either retained or re-expressed on a small subset (5%-10%) of the expanded effector population, and these cells go on to up-regulate bcl-2, thereby escaping apoptosis and allowing their selection into long-term memory.4,11,13 Thus, in mice, both IL-7 and IL-15 appear to be playing important complementary roles at different stages of the T-cell response to infection with intracellular pathogens.4,12,14
Here, we have used the model of Epstein-Barr virus (EBV) infection to ask to what extent such findings mirror events occurring during the human T-cell response to viral challenge. Primary EBV infection, as seen in infectious mononucleosis (IM) patients, is associated with a marked CD8+ T-cell expansion that is largely virus-specific and has been well characterized in terms of immunodominant EBV lytic and latent cycle epitopes.15,16 We therefore followed IM patients prospectively, from the time of acute IM through convalescence into the asymptomatic virus carrier state, and characterized both EBV-specific and total T-cell population for cytokine receptor expression and for cytokine responsiveness.
Patients, materials, and methods
Donors
Cases of IM were identified on clinical grounds and confirmed by high leukocyte counts. EBV-associated cases were identified by heterophile antibody positivity and high EBV DNA loads in peripheral blood mononuclear cells (PBMCs). Cytomegalovirus (CMV)-associated cases were identified among heterophile antibody-negative adult mononucleosis patients by the presence of CMV-specific IgM antibodies, with subsequent isotype switching to IgG, and high CMV DNA loads in plasma.17 Individual IM patients were bled in the acute phase and/or at different time points after infection (between 1 month and 14 years); all patients included in the study had shown resolution of acute disease symptoms within 4 weeks of the initial acute phase. Blood samples were also taken from healthy donors, most of whom were in the same age range (18-30 years) as the EBV-IM patients. These included 30 individuals known to have been EBV and/or CMV carriers for at least 5 years and to have no prior history of IM, 30 individuals with no serologic evidence of prior EBV infection, and 5 individuals with no serologic evidence of prior CMV infection. In each case, PBMCs were isolated from heparinized blood and aliquots of cells were cryopreserved. Ethics approval for this study was granted from the South Birmingham Health Authority Local Research Ethics Committee.
Tetramers
HLA class I tetramers were prepared for the following epitopes/HLA combinations identified in earlier work15,18,19 : EBV lytic cycle epitopes: GLCTLVAML/HLA-A*0201, YVLDHLIVV/HLA-A*0201, RAKFKQLL/HLA-B*0801, and EPLPQGQLTAY/HLA-B*3501; EBV latent cycle epitopes: CLGGLLTMV/HLA-A*0201, FLRGRAYGL/HLA-B*0801, QAKWRLQTL/HLA-B*0801, YPLHEQHGM/HLA-B*3501, and HPVGE-ADYFEY/HLA-B*3501; and CMV epitopes: NLVPMVATV/HLA-A*0201, VLEETSVML/HLA-A*0201, ELRRKMMYM/HLA-B*0801, ELKRK-MIYM/HLA-B*0801, QIKVRVDMV/HLA-B*0801, IPSINVHHY/HLA-B*3501, and TPRVTGGGAM/HLA-B*0701. Throughout this paper, the nomenclature for the epitopes has been abbreviated to the first 3 amino acids (as underlined in the preceding sentence). Peptides were purchased from Alta Biosciences (University of Birmingham, Birmingham, United Kingdom) and phycoerythrin (PE)-conjugated tetramers were produced as described.15
Cell staining
In stainings that included tetramers, cells were first incubated with a pretitrated concentration of PE-conjugated tetramer (∼ 0.5 μg/mL) at 37°C for 15 minutes and then washed, and all further steps were conducted on ice. Cells were exposed to human IL-Rα-specific antibodies (below) for 30 minutes, followed by the appropriate fluorescein isothiocyanate (FITC) secondary antibodies for another 30 minutes; cells were then blocked with mouse serum (Dako, Glostrup, Denmark) and finally stained with a Tricolour-labeled anti-human CD8 monoclonal antibody (mAb; Caltag Laboratories, Burlingame, CA) for 30 minutes. Staining was analyzed on an Epics flow cytometer (Beckman Coulter, Fullerton, CA). Goat anti-human IL-7Rα antibody (R&D Systems, Minneapolis, MN) was detected by FITC-conjugated swine anti-goat IgG antibody (Caltag Laboratories); mouse anti-human IL-15Rα (clone 151307; R&D systems) was detected by FITC-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates, Birmingham, AL). As control, a FITC-conjugated IgG1 mouse isotype control was used followed by the above FITC-conjugated secondary reagent.
In examining mononuclear cell subsets for receptor expression, PBMCs were first exposed to human IL-Rα-specific antibodies followed by a FITC-conjugated second step reagent, then washed and stained for 30 minutes on ice with either Tricolour-labeled CD8- or CD4-specific mAbs (Caltag Laboratories) or PE-labeled CD56-, CD19-, and CD14-specific mAbs (Immunotech, Marseille, France), and staining was analyzed by flow cytometry. In confirmatory experiments (in combination with CD8 and CD4 stainings), we used a second IL-15Rα mAb (clone 151303; R&D Systems).
IL-7- and IL-15-induced STAT5 phosphorylation
PBMCs were exposed to PE-labeled tetramer for 15 minutes at 37°C in the presence of recombinant IL-7 or IL-15 (R&D Systems) at doses up to 100 ng/mL, then washed and fixed in 2% formaldehyde in phosphate-buffered saline (PBS; Gibco, Paisley, United Kingdom) for 10 minutes at room temperature. Cells were subsequently stained with Tricolour-labeled anti-CD8, then permeabilized with ice-cold 90% methanol, stained intracellularly for 30 minutes at room temperature using rabbit anti-human phospho STAT5 mAb (Tyr694; Cell Signaling Technology, Beverly, MA) followed by FITC-conjugated goat anti-rabbit IgG (Southern Biotechnology Associates), and analyzed by flow cytometry. In some cases, IL-15-induced STAT5 phosphorylation of total CD8+ T cells was assayed as described on PBMCs and in parallel on CD8+ purified T cells and CD14-depleted PBMCs from the same donors (Miltenyi Biotech, Bergisch Gladbach, Germany). CD8+ T cells were purified by positive selection (CD8 microbeads; Miltenyi Biotech), and CD14+ cell depletion was performed using mAb-coated magnetic beads according to the manufacturer's instructions (CD14 microbeads; Miltenyi Biotech).
IL-7- and IL-15-supplemented PBMC cultures
PBMCs were resuspended in culture medium, consisting of RPMI (Gibco), 10% vol/vol fetal calf serum (FCS; Gibco), and recombinant human IL-7 or IL-15 (R&D Systems) at a final concentration of 1 ng/mL, with or without the addition of anti-CD3/CD28 coated-beads (1 bead/cell; Dynal, Compiegne, France) as a costimulus. Cells were counted at days 3, 5, and 7 and each time recultured at 1 × 106 cells/mL; a final count was made at day 10. Levels of IL-7Rα and IL-15Rα on cells were determined on the starting population and on days 3, 7, and 10 by costaining as described under “Cell staining.”
Statistical analysis
Statistical analysis was performed using GraphPad prism software (San Diego, CA). Data were compared using a Mann-Whitney test and significant differences verified with 95% confidence intervals.
Results
IL-7Rα and IL-15Rα expression on EBV-specific CD8+ T cells
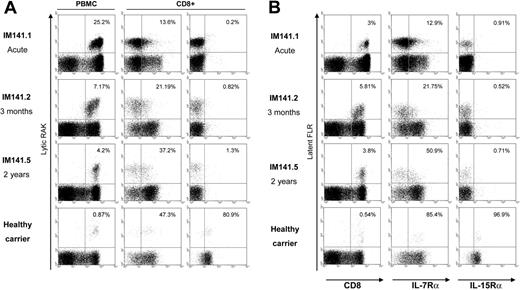
Figure 1 shows data from a typical experiment comparing an IM patient, IM141, studied in the acute phase of disease and again 3 months and 2 years later, with a healthy HLA-B*0801-matched EBV carrier who had no history of IM. Tetramer staining identifies CD8+ T cells reactive to 2 B*0801-restricted viral epitopes, the lytic epitope RAK (Figure 1A) and the latent epitope FLR (Figure 1B), and reveals degrees of expansion and subsequent contraction of these virus-specific responses that are typical of primary EBV infection.15 For both epitope-specific populations, IL-7Rα expression was absent in the acute IM bleed, but thereafter the proportion of IL-7Rα-positive cells gradually increased toward the levels seen on the corresponding memory populations in the healthy carrier. By contrast, IL-15Rα expression was undetectable on epitope-specific cells in acute IM but remained so in the 2 later bleeds. In the healthy carrier, this was markedly different from epitope-specific memory cells, which were largely (> 80%) IL-15Rα positive.
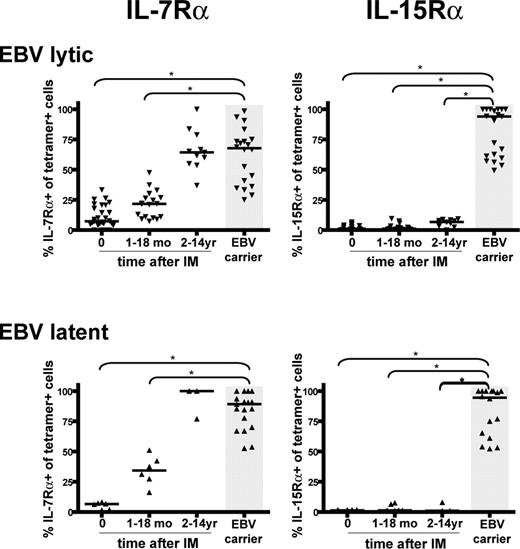
Figure 2 summarizes the cumulative results on EBV-specific CD8+ T cells from 8 IM patients followed prospectively from the time of acute infection, from another 15 IM patients studied only in the acute phase of IM, and from 22 IM patients from whom only later bleeds (1 to 14 years after IM) were available. They are compared with data from 22 healthy EBV carriers, none of whom had any history of symptomatic primary EBV infection. Overall, the phenotypes of 5 EBV lytic and 5 EBV latent epitopes, restricted through either HLA-A*0201, B*0801, or B*3501, were examined. All were consistently low for IL-7Rα expression in acute IM, after which expression slowly increased, eventually reaching the same range as seen in healthy carriers but only after a period of 2 or more years. Generally, latent epitope-specific memory populations showed a higher percentage of IL-7Rα-positive cells (median = 89.1%) than lytic epitope-specific memory (median = 67.7%, P = .002). By contrast, IL-15Rα expression remained essentially undetectable on EBV lytic and latent epitope-specific memory in all post-IM patients, whereas expression was high (medians of 93.9% and 94.5% respectively, P < .001) on the equivalent memory cells in healthy carriers.
IL-7Rα and IL-15Rα expression on EBV-specific CD8+ T cells. Representative staining profiles are shown for CD8+ T cells specific for (A) the HLA-B*0801-restricted EBV lytic cycle epitope RAK and (B) for the HLA-B*0801-restricted EBV latent cycle epitope, FLR. (Left panels) Flow cytometry profiles of tetramer versus CD8 staining among PBMCs; percentage values refer to the percentage of tetramer-positive cells in the CD8+ population. (Middle and right panels) Flow cytometry profiles of tetramer versus IL-7Rα and tetramer versus IL-15Rα staining among CD8+ T cells, respectively; percentage values refer to the percentage of IL-Rα-positive cells within the tetramer-positive population. From top to bottom, panels show the profiles obtained from one HLA-B*0801-positive patient, IM141, studied in acute phase (IM141.1), 3 months later (IM141.2), and 2 years later (IM141.5), all stained in the same experiment alongside cells from an HLA-B*0801-positive healthy EBV carrier with no history of IM.
IL-7Rα and IL-15Rα expression on EBV-specific CD8+ T cells. Representative staining profiles are shown for CD8+ T cells specific for (A) the HLA-B*0801-restricted EBV lytic cycle epitope RAK and (B) for the HLA-B*0801-restricted EBV latent cycle epitope, FLR. (Left panels) Flow cytometry profiles of tetramer versus CD8 staining among PBMCs; percentage values refer to the percentage of tetramer-positive cells in the CD8+ population. (Middle and right panels) Flow cytometry profiles of tetramer versus IL-7Rα and tetramer versus IL-15Rα staining among CD8+ T cells, respectively; percentage values refer to the percentage of IL-Rα-positive cells within the tetramer-positive population. From top to bottom, panels show the profiles obtained from one HLA-B*0801-positive patient, IM141, studied in acute phase (IM141.1), 3 months later (IM141.2), and 2 years later (IM141.5), all stained in the same experiment alongside cells from an HLA-B*0801-positive healthy EBV carrier with no history of IM.
IL-7Rα and IL-15Rα expression on PBMC cell subsets
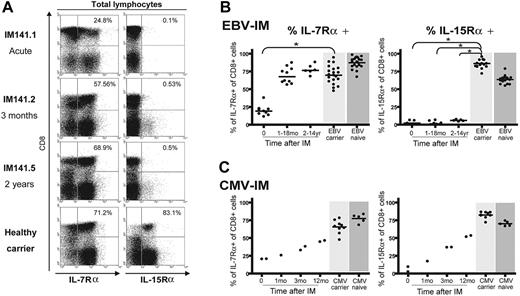
We noted from Figure 1 that the absence of IL-15Rα after IM was not limited to the EBV-specific CD8 response but appeared to affect the whole CD8+ T-cell population. Figure 3A shows IL-7Rα and IL-15Rα staining for the total peripheral blood lymphocyte population of patient IM141 at 3 different time points, again compared with a healthy EBV carrier control. In the acute IM sample, most (75%) of the expanded CD8+ T-cell population was IL-7Rα negative. However, after the resolution of symptoms and the return to a normal CD4/CD8 ratio in the blood, the total CD8+ T-cell pool had recovered IL-7Rα expression; note that the CD8- lymphocyte population (mainly CD4+ T cells) remained predominantly IL-7Rα positive at all times. By contrast, the total CD8+ T-cell pool, and the majority of cells in the non-CD8+ population, was IL-15Rα negative both in acute IM and up to 2 years later, quite different from the profile seen in a healthy carrier. Figure 3B summarizes the overall results from such experiments. Following IM, IL-7Rα expression on total CD8+ T cells recovers to healthy control donor values within a matter of months, whereas after IM CD8+ T cells remained IL-15Rα negative at all time points studied up to 14 years after disease. In view of these findings, we considered the possibility that this IL-15Rα deficit might exist in certain individuals before primary EBV infection and that this could predispose them to IM. However, we analyzed PBMC samples from 30 healthy EBV-naive adults and found that all expressed both IL-7Rα and IL-15Rα on the majority of CD8+ T cells (Figure 3B).
Summary of IL-7Rα and IL-15Rα expression on EBV-specific CD8+ T cells in acute IM and post-IM patients versus healthy carriers. Scatterplots of IL-7Rα expression or IL-15Rα on EBV lytic epitope-specific CD8+ T cells (top panels) and on EBV latent epitope-specific CD8+ cells (bottom panels). Results are expressed as the percentage of tetramer-positive cells that are IL-7Rα (left panels) or IL-15Rα (right panels) positive, and each individual symbol shows the value for a particular epitope in a particular donor tested within a particular time interval after infection (ie, in acute IM, 1-18 months after IM, and 2-14 years after IM). Results for equivalent EBV epitope-specific memory cells in healthy EBV carriers are also shown (gray area). Horizontal lines represent the median value for each specificity/time-point combination. Statistical analysis was performed using the Mann-Whitney test; significant differences are indicated by asterisks (*P < .001).
Summary of IL-7Rα and IL-15Rα expression on EBV-specific CD8+ T cells in acute IM and post-IM patients versus healthy carriers. Scatterplots of IL-7Rα expression or IL-15Rα on EBV lytic epitope-specific CD8+ T cells (top panels) and on EBV latent epitope-specific CD8+ cells (bottom panels). Results are expressed as the percentage of tetramer-positive cells that are IL-7Rα (left panels) or IL-15Rα (right panels) positive, and each individual symbol shows the value for a particular epitope in a particular donor tested within a particular time interval after infection (ie, in acute IM, 1-18 months after IM, and 2-14 years after IM). Results for equivalent EBV epitope-specific memory cells in healthy EBV carriers are also shown (gray area). Horizontal lines represent the median value for each specificity/time-point combination. Statistical analysis was performed using the Mann-Whitney test; significant differences are indicated by asterisks (*P < .001).
To ask whether other pathogens inducing clinically apparent mononucleosis might likewise have long-lasting effects on cytokine receptor expression, we studied 4 individuals who presented with severe IM-like symptoms and large CD8+ T-cell expansions caused by primary CMV infection. As shown in Figure 3C, recovery from acute primary CMV infection was accompanied by rising levels of both IL-7Rα and IL-15Rα on total CD8+ T cells, approaching the levels seen in healthy CMV carriers and in CMV-naive individuals. In one of these CMV-IM patients, we were able to study the CD8 response to a CMV-coded epitope (the B7-restricted lytic epitope TPR from the pp65 protein) in acute phase and at 3 and 12 months later. As shown in Figure S1 (available on the Blood website; see the Supplemental Figures link at the top of the online article), TPR-specific T cells gradually recovered expression of both cytokine receptors, following the trend seen for receptor expression on CD8+ T cells as a whole. These findings strongly suggest that the long-term deficit in IL-15Rα expression seen following classic IM is not a consequence of CD8+ T-cell hyperexpansion, per se, but is a specific marker of individuals with a history of the EBV-associated disease.
IL-7Rα and IL-15Rα expression on total lymphocyte populations. (A) Representative staining profiles for IL-7Rα expression (left) and IL-15Rα expression (right) on PBMCs costained with anti-CD8 mAb. Analysis was restricted to the lymphocyte population (ie, excluding monocytes) by gating on cell size. These results involve the same IM donor, studied in acute phase, 3 months later, and 2 years later, and the same healthy EBV carrier as in Figures 1 and 2. Percentage values refer to the percentage of IL-R-positive cells among the CD8+ population. (B-C) Scatterplots summarizing IL-7Rα expression (left panel) and IL-15Rα expression (right panel) on total CD8+ T cells during and at intervals after EBV-associated IM (B) or CMV-associated IM (C). In all plots, results are expressed as the percentage of CD8+ cells that are IL-7Rα or IL-15Rα positive. Each symbol shows the value for a particular donor within a particular time interval after infection. Cumulative results from IL-R stainings carried out on the CD8+ T cells of 22 healthy EBV carriers with no history of EBV-IM (light gray shading) and 30 healthy EBV-naive individuals (dark gray shading) are shown in the top panels; similarly, results from 10 healthy CMV carriers and 5 healthy CMV-naive individuals are shown in the bottom panels. Horizontal lines represent the median value in each case. Statistical analysis was performed using the Mann-Whitney test; significant differences are indicated by asterisks (*P < .001).
IL-7Rα and IL-15Rα expression on total lymphocyte populations. (A) Representative staining profiles for IL-7Rα expression (left) and IL-15Rα expression (right) on PBMCs costained with anti-CD8 mAb. Analysis was restricted to the lymphocyte population (ie, excluding monocytes) by gating on cell size. These results involve the same IM donor, studied in acute phase, 3 months later, and 2 years later, and the same healthy EBV carrier as in Figures 1 and 2. Percentage values refer to the percentage of IL-R-positive cells among the CD8+ population. (B-C) Scatterplots summarizing IL-7Rα expression (left panel) and IL-15Rα expression (right panel) on total CD8+ T cells during and at intervals after EBV-associated IM (B) or CMV-associated IM (C). In all plots, results are expressed as the percentage of CD8+ cells that are IL-7Rα or IL-15Rα positive. Each symbol shows the value for a particular donor within a particular time interval after infection. Cumulative results from IL-R stainings carried out on the CD8+ T cells of 22 healthy EBV carriers with no history of EBV-IM (light gray shading) and 30 healthy EBV-naive individuals (dark gray shading) are shown in the top panels; similarly, results from 10 healthy CMV carriers and 5 healthy CMV-naive individuals are shown in the bottom panels. Horizontal lines represent the median value in each case. Statistical analysis was performed using the Mann-Whitney test; significant differences are indicated by asterisks (*P < .001).
From IL-15Rα staining profiles such as Figure 3A, the lymphocyte pool of post-IM patients contained a small subset of CD8- cells that were IL-15Rα+. To identify these cells, we costained unfractionated PBMCs for the receptor and for subset markers. Data from 2 post-IM patients (studied 5 and 14 years after their acute infection) and from 2 healthy EBV carriers are shown in Figure 4. The post-IM patients expressed IL-15Rα on both CD19+ B cells and CD14+ monocytes at levels equivalent to that seen in healthy carriers, but failed to express IL-15Rα on CD8+ T cells, CD4+ T cells, or CD56+ natural killer (NK) cells. We consistently observed these differences in assays on PBMCs from 11 post-IM patients versus 9 healthy carriers, using 2 different anti-IL-15Rα-specific mAbs.
Expression of IL-15Rα on different PBMC cell subsets. Histograms of IL-15Rα staining are shown for 2 long-term post-IM patients studied 5 and 14 years after infection (left panel) and for 2 healthy EBV carriers (right panel); in each case, PBMCs were costained with IL-15Rα mAb and with cell subset-specific markers. From top to bottom, panels show IL-15Rα staining profiles on gated populations of CD8+ T cells, CD4+ T cells, CD56+ NK cells, CD19+ B lymphocytes, and CD14+ monocytes. The shaded histograms represent staining of the same gated population with a mouse isotype control. These results are representative of those obtained in experiments on 11 post-IM patients and 9 healthy carriers.
Expression of IL-15Rα on different PBMC cell subsets. Histograms of IL-15Rα staining are shown for 2 long-term post-IM patients studied 5 and 14 years after infection (left panel) and for 2 healthy EBV carriers (right panel); in each case, PBMCs were costained with IL-15Rα mAb and with cell subset-specific markers. From top to bottom, panels show IL-15Rα staining profiles on gated populations of CD8+ T cells, CD4+ T cells, CD56+ NK cells, CD19+ B lymphocytes, and CD14+ monocytes. The shaded histograms represent staining of the same gated population with a mouse isotype control. These results are representative of those obtained in experiments on 11 post-IM patients and 9 healthy carriers.
Functional CD8+ T-cell responses to IL-7 and IL-15 in relation to receptor status
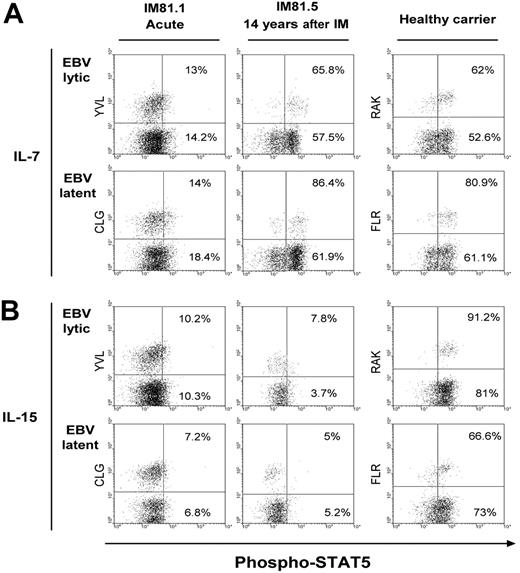
To determine whether cytokine receptor expression levels correlated with responsiveness, we exposed PBMCs from post-IM patients and healthy virus carriers to 1 ng/mL IL-7 or IL-15 and then assayed for cytokine-triggered phosphorylation of STAT5 as a marker of response. Figure 5 shows data from a representative IM patient, the HLA-A*0201-positive IM81, studied in acute phase and 14 years after infection, and from an HLA-B*0801-positive EBV carrier control. Responses to IL-7 among CD8+ T cells (Figure 5A) were barely detectable in the acute IM81.1 sample, whether focusing on cells specific for EBV lytic (YVL) or latent (CLG) epitopes or on the total CD8 population. However, in the IM81.5 sample taken 14 years later, the majority of cells in both epitope-specific memory populations and in the CD8 population as a whole showed STAT5 phosphorylation at levels indistinguishable from the healthy carrier. Of importance, parallel assays using IL-15 as the stimulus (Figure 5B) clearly showed that the absence of IL-15Rα staining on acute IM and post-IM T cells was reflected in the lack of a detectable response to the cytokine, whereas responses were clearly observed in cells of the healthy carrier. These differences in IL-15 responsiveness were consistently observed in assays comparing 5 acute IM and 8 post-IM donors with 14 healthy EBV carriers.
Cytokine-induced STAT5 phosphorylation in CD8+ populations. Representative example of STAT5 phosphorylation induced after stimulation of PBMCs with (A) 1 ng/mL IL-7 or (B) 1 ng/mL IL-15. Results are shown for the HLA-A*0201-positive patient IM81 studied in the acute disease (IM81.1) and 14 years later (IM81.5), and for an HLA-B*0801-positive healthy carrier. In each case, STAT5 phosphorylation is analyzed on EBV lytic epitope-specific populations (against the HLA-A*0201-restricted YVL or HLA-B*0801-restricted RAK epitope) and EBV latent epitope-specific populations (against the HLA-A*0201-restricted CLG or HLA-B*0801-restricted FLR epitopes). Percentage values in the top right quadrant of each dot plot refer to the percentage of STAT5 phosphorylation in tetramer-positive cells, whereas values in the bottom right quadrant indicate the percentage of STAT5 phosphorylation in the total CD8+ population. Quadrant boundaries were set using nonstimulated cells from the same donors.
Cytokine-induced STAT5 phosphorylation in CD8+ populations. Representative example of STAT5 phosphorylation induced after stimulation of PBMCs with (A) 1 ng/mL IL-7 or (B) 1 ng/mL IL-15. Results are shown for the HLA-A*0201-positive patient IM81 studied in the acute disease (IM81.1) and 14 years later (IM81.5), and for an HLA-B*0801-positive healthy carrier. In each case, STAT5 phosphorylation is analyzed on EBV lytic epitope-specific populations (against the HLA-A*0201-restricted YVL or HLA-B*0801-restricted RAK epitope) and EBV latent epitope-specific populations (against the HLA-A*0201-restricted CLG or HLA-B*0801-restricted FLR epitopes). Percentage values in the top right quadrant of each dot plot refer to the percentage of STAT5 phosphorylation in tetramer-positive cells, whereas values in the bottom right quadrant indicate the percentage of STAT5 phosphorylation in the total CD8+ population. Quadrant boundaries were set using nonstimulated cells from the same donors.
Figure 6 summarizes data from further experiments comparing responses (in 11 long-term post-IM patients versus 18 healthy carrier controls) to increasing cytokine concentrations, mean levels of response being expressed in each case as the percentage of cells showing STAT5 phosphorylation in EBV epitope-specific and in total CD8+ T-cell populations. Responses to IL-7 (Figure 6A) titrated similarly in the 2 types of donor, with responses just detectable at 0.01 ng/mL IL-7 and peaking at 10 to 100 ng/mL; the only discernable difference was that the maximal response in the total CD8 T-cell population was consistently lower in post-IM patients than in healthy carriers. However, responses to IL-15 were quite different (Figure 6B): while healthy carriers gave titration curves similar to those seen for IL-7, with significant levels of STAT5 phosphorylation detectable at 0.1 ng/mL IL-15 and almost maximal levels at 10 ng/mL, the post-IM donors began to show some responses only at 10 ng/mL or higher. From these titration curves, estimates of cytokine doses required for half-maximal responses showed that post-IM donor CD8+ T cells (whether the EBV-specific or the total population) were more than 20-fold less sensitive to IL-15 than healthy carrier cells. This is consistent with IL-15Rα-negative post-IM CD8+ T cells binding cytokine via the low-affinity β/γ complex of the IL-15R.7 Indeed, we confirmed by specific mAb staining that CD8+ T cells from post-IM donors did express both β and γ chains at levels in the same range as CD8+ T cells from healthy carriers (data not shown).
Note that these previous assays were conducted on whole PBMC populations that contain monocytes. This was a potential concern since, in mouse systems, monocytes have been shown to bind IL-15 through their expression of the IL-15Rα chain and transpresent the cytokine to CD8+ T cells via the low-affinity βγ receptor on the T-cell surface.10,20-23 We therefore repeated the above titrations using both monocyte-depleted PBMCs and purified CD8+ T cells as responder populations. As shown in Figure 6C, the clear differential between post-IM and healthy carrier responses was maintained whether or not accessory cells were present. These findings strongly suggest that, in this in vitro system, responses are being induced by direct IL-15 engagement of CD8+ T cells and that the impaired responsiveness of post-IM CD8+ T cells is a direct consequence of IL-15Rα down-regulation on these cells.
Titration of STAT5 phosphorylation response to IL-7 and IL-15. (A-B) Mean results from 11 post-IM patients (closed symbols) and 18 healthy carriers (open symbols) in experiments assaying cytokine-induced STAT5 phosphorylation after exposure to the indicated doses of (A) IL-7 or (B) IL-15. Results are expressed as the percentage of EBV tetramer-positive cells (left panels) or total CD8+ cells (right panels) showing STAT5 phosphorylation. Data on EBV-specific populations represent cumulative results obtained with 4 tetramers all specific for EBV lytic cycle epitopes; similar results were obtained with 5 latent epitope-specific tetramers. (C) STAT5 phosphorylation in assays conducted on purified CD8+ populations (left panel) and on CD14-depleted PBMC populations (right panel). Representative results are shown from 1 of 5 experiments comparing post-IM patient and healthy carrier responses to the indicated doses of IL-15; results are expressed as the percentage of total CD8+ T cells showing STAT5 phosphorylation.
Titration of STAT5 phosphorylation response to IL-7 and IL-15. (A-B) Mean results from 11 post-IM patients (closed symbols) and 18 healthy carriers (open symbols) in experiments assaying cytokine-induced STAT5 phosphorylation after exposure to the indicated doses of (A) IL-7 or (B) IL-15. Results are expressed as the percentage of EBV tetramer-positive cells (left panels) or total CD8+ cells (right panels) showing STAT5 phosphorylation. Data on EBV-specific populations represent cumulative results obtained with 4 tetramers all specific for EBV lytic cycle epitopes; similar results were obtained with 5 latent epitope-specific tetramers. (C) STAT5 phosphorylation in assays conducted on purified CD8+ populations (left panel) and on CD14-depleted PBMC populations (right panel). Representative results are shown from 1 of 5 experiments comparing post-IM patient and healthy carrier responses to the indicated doses of IL-15; results are expressed as the percentage of total CD8+ T cells showing STAT5 phosphorylation.
Finally, we studied the in vitro proliferative response to 1 ng/mL IL-7 and IL-15, comparing PBMCs from long-term post-IM patients with those from healthy carriers, in each case with or without anti-CD3/CD28 mAb-coated beads as a stimulus. As shown in Figure 7A, there was a detectable proliferative response to IL-7 in post-IM PBMC cultures, with or without CD3/CD28 costimulation, although this was reproducibly less than that observed in healthy carriers (Figure 7A). However, while PBMCs from healthy carriers maintained cell numbers when cultured in IL-15 alone and proliferated well in costimulated cultures, in both situations post-IM PBMCs showed significant levels of cell death within the first 3 days; thereafter, there was some proliferation in costimulated cultures but progressive death in IL-15 alone (Figure 7B). In the same experiments, we monitored cytokine receptor expression at the beginning of cell culture and in viable cells harvested 3 and 10 days after CD3/CD28 costimulation in the presence of cytokine. As shown in Figure S2A, in both post-IM and healthy carrier cultures, expression of IL-7Rα was rapidly down-regulated on CD8+ and on CD8- lymphocyte populations by day 3, but then recovered to reach high levels by day 10; at that time, the majority of cells in the cultures were in fact CD8+ T cells. In the IL-15 and CD3/CD28-costimulated cultures, the healthy carrier showed a similar response, with down-regulation of IL-15Rα expression by day 3 followed by recovery at day 10. However, even after such combined cytokine and CD3/CD28 costimulation, there was never any induction of IL-15Rα on cells from post-IM donors (Figure S2B).
Cell numbers in IL-7- and IL-15-supplemented PBMC cultures. Mean results from 8 post-IM patients and 6 healthy EBV carriers in experiments where PBMCs were seeded in culture at 1 × 106/mL in the presence of (A) IL-7 or (B) IL-15 at 1 ng/mL, with or without anti-CD3/CD28-coated beads as a costimulus. Cell counts were performed on days 3, 5, 7, and 10, and viable cell numbers are expressed relative to the initial seeding.
Cell numbers in IL-7- and IL-15-supplemented PBMC cultures. Mean results from 8 post-IM patients and 6 healthy EBV carriers in experiments where PBMCs were seeded in culture at 1 × 106/mL in the presence of (A) IL-7 or (B) IL-15 at 1 ng/mL, with or without anti-CD3/CD28-coated beads as a costimulus. Cell counts were performed on days 3, 5, 7, and 10, and viable cell numbers are expressed relative to the initial seeding.
Discussion
Prompted by studies showing a role for IL-7 and IL-15 in the development and maintenance of CD8+ memory T cells to viral infection in mouse systems,4,24 the present work explored the analogous situation in humans by following young adults in whom primary EBV infection is manifest as IM. With regard to IL-7, as for the primary responses in mice,11 most of the highly expanded CD8+ T-cell population seen in the blood of acute IM patients, including defined EBV epitope-specific populations, has down-regulated IL-7Rα expression. Thereafter, IL-7Rα expression on the total CD8 population recovered to healthy control donor levels within a few months, by which time activated cells have disappeared from the blood and the circulating CD8+ T-cell pool has returned within the normal range.15,25 Of interest, EBV epitope-specific CD8+ T cells in the blood of post-IM patients take 2 or more years to acquire the levels of IL-7Rα expression shown by equivalent memory populations in healthy EBV carriers. This contrasts with the situation in mice that have controlled and cleared lymphocytic choriomeningitis virus (LCMV) infection, where IL-7Rα up-regulation appears to be an immediate identifier of cells entering memory.4,11,13 The data from post-IM patients are in fact closer to those reported for mice carrying persistent low-grade LCMV infection, where virus-specific CD8+ T cells express only low levels of IL-7Rα and appear to be dependent upon continual antigenic stimulation rather than upon IL-7-mediated homeostatic signals for their maintenance.26
Parallels with mouse models broke down when the study turned to IL-15Rα expression. First, while mouse CD8+ T cells are reported to increase IL-15Rα levels in response to antigen stimulation,4,10 we found that IL-15Rα was down-regulated on all activated CD8+ T cells that dominate the blood picture in acute IM, including identifiable EBV epitope-specific populations. Second, and most surprising, IL-15Rα remained undetectable on CD8+ T cells (again including EBV-specific T cells), on CD4+ T cells, and on CD56+ NK cells long after recovery from acute IM. This global deficit in IL-15Rα expression was a defining characteristic of all 22 post-IM patients studied up to 14 years after infection. By contrast, 22 healthy EBV carriers who had no history of IM (but were age-matched with the IM cohort) always showed IL-15Rα staining on circulating T- and NK-cell populations. Although there is no evidence for any familial predisposition to acute IM (except in the special case of SH2D1A mutation27 ), the data forced us to consider the possibility that an IL-15Rα deficit pre-existed in a subset of EBV-naive adults and that this was associated with a unique susceptibility to symptomatic primary infection. Unfortunately, we could not trace any IM patients from whom PBMCs had been stored before their disease episode. As an alternative, we screened a total of 30 EBV-naive donors (most of whom were young adults) and found that all expressed IL-15Rα on T cells. Since 25% to 50% of individuals contracting primary EBV infection as adults are reported to develop IM symptoms,28,29 any deficit predisposing to IM should have been seen in some members of this cohort. The results strongly suggest that IL-15Rα down-regulation is a consequence (not a precondition) of EBV-associated IM. We then asked whether the effect extended to patients with a clinical history of mononucleosis, associated with large CD8+ T-cell expansions, caused by primary CMV infection. While IL-15Rα (and to a lesser extent IL-7Rα) was down-regulated in acute CMV-IM, there was clearly a recovery of receptor expression on total and on CMV-specific CD8+ T cells over the ensuing 12 months. Thus, hyperexpansion of the CD8+ T-cell pool is itself not sufficient to explain the post-EBV-IM phenotype.
These findings raised the possibility that transient downregulation of IL-15Rα (and to a lesser extent IL-7Rα) expression on CD8+ T cells might be a general feature of acute virus infections in humans. In support of this, we recently studied 2 cases of flulike respiratory tract infection that occurred among our healthy EBV carrier cohort and were associated with transient CD8+ T-cell expansions reaching 38% and 41% of the total PBMC population. These individuals indeed showed down-regulation of IL-15Rα expression on all circulating CD8+ T cells at the height of disease, yet within 2 weeks of the resolution of symptoms, IL-15Rα levels had recovered to those seen before symptoms arose. Expression of IL-7Rα was also reduced during the disease episode and reappeared within one week of clinical recovery (D.S. and M.L., unpublished results, July 2005). We infer that such global downregulation of both receptors might be part of an innate physiologic response to acute viral infection, perhaps induced by one or more cytokines produced during such infection. In considering what advantage such a response might bring, it is worth noting that the main effects of IL-7 and IL-15 appear to be in relation to T-cell homeostasis rather than in the regulation of antigen-driven T-cell activation per se.24,26,30,31 Indeed, combinations of IL-7 and IL-15 are reported to have global effects on human CD8+ T cells in vitro, driving CCR7+, CD45RO+ central memory cells to acquire CCR7-, CD45RO+ effector memory or CCR7-, CD45RA+ effector phenotypes.32 This has been proposed as a mechanism in vivo for slow antigen-independent replenishment of effector cell populations from the central memory pool.33 Transient down-regulation of the IL-7 and IL-15 receptors on T cells during an acute viral infection might therefore guard against the central memory repertoire being depleted of reactivities irrelevant to the immediate viral challenge.
Acute primary EBV infection, when manifest as IM, appears to be unique in producing a global down-regulation of IL-15Rα on T cells and NK cells that lasts for many years after the disease episode. Whether this long-term receptor deficit has any functional consequences for IL-15 responsiveness became a key question. Thus, in mouse systems there is very strong evidence that IL-15Rα status is not the major determinant of a CD8+ T cell's ability to respond to IL-15. Rather, monocytes are able to capture IL-15 on their surface through expression of IL-15Rα chain and transpresent the cytokine to CD8+ T cells via the latter's low-affinity IL-15Rβ/γ complex.20-23,34 We therefore compared the cytokine responsiveness of post-IM and healthy carrier CD8+ T cells by assaying IL-15-induced STAT5 phosphorylation in vitro in the presence and absence of accessory cells. In both situations, post-IM donors were markedly impaired in their response. This functional defect correlated with the absence of IL-15Rα on post-IM T cells and not with other components of the IL-15R since expression of the IL-15Rβ and γ chains was equivalent in post-IM and healthy carrier cohorts. Such findings imply that, in humans, IL-15Rα status may be a more important determinant of CD8+ T-cell sensitivity to IL-15 than it is in mice. We infer that, at least in our in vitro system, transpresentation by accessory cells is not the principal pathway of IL-15 acquisition by human CD8+ T cells.
These in vitro studies leave open the question as to what the biologic consequences of IL-15Rα down-regulation might be in vivo. Ostensibly post-IM patients maintain EBV-specific CD8+ T-cell numbers in the memory pool15,35 without those cells expressing the high-affinity IL-15Rα. This could be due to compensatory effects mediated by other cytokines or it could reflect a situation such as that seen in mice carrying the murine γ-herpesvirus MHV68, where the persistence of virus-specific memory appears to be dependent not upon cytokine signaling but upon chronic antigenic stimulation from the virus.36 We would also stress that while EBV-specific T cells are present in IM and post-IM patients, we do not know how biologically effective such responses are. Thus, IM itself can be a protracted disease with recurrent symptoms in severe cases37 ; even in uncomplicated cases, the EBV-host balance remains abnormal for long periods after disease resolution38,39 and may never reach that typically struck after asymptomatic primary infection. Furthermore, recent epidemiologic evidence has shown that post-IM patients remain at significantly increased risk of an EBV-positive malignancy, Hodgkin lymphoma, for at least 10 years after their original disease episode.40 Given the widespread nature of the IL-15Rα down-regulation on T and NK cells, and the possibility that some nonhematopoietic cell lineages4 could also be affected, a history of IM may carry with it other disease risks that have not yet been recognized. These longer-term issues remain to be resolved. More immediately, the present work shows that symptomatic primary EBV infection leaves a longlasting scar on the immune system. As a result, post-IM patients represent a potentially unique cohort of individuals through which to analyze the effects of impaired IL-15 signaling not just on human T-cell and NK-cell functions in vitro but also on the homeostatic turnover of these cell populations in vivo.41
Prepublished online as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2006-01-0144.
Supported by the Medical Research Council, United Kingdom. D.S. has benefited from a Lavoisier grant from the Ministère des affaires étrangères, France.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are very grateful to Dr Naeem Khan (Birmingham) for providing CMV-restricted tetramers; to Dr Mark Wills (Cambridge) for access to PBMCs from post-CMV-IM patients; and to Drs Dorothy Crawford (Edinburgh), Jan Gratama (Rotterdam), Eric Robinet (Besançon), Cliona Rooney (Houston), and Tim Wellinger (Oxford) for providing PBMCs from EBV-naive donors.
The authors have no conflicting financial interests.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal