Ventricular tachyarrhythmias may occur during intravenous arsenic trioxide (As2O3). This has not happened during oral As2O3. Sixteen patients were studied by electrocardiography and 24-hour Holter monitoring at baseline, during and after oral As2O3 (As2O3-ON, As2O3-OFF). QT and corrected QT (QTc) were significantly longer during As2O3-ON than in As2O3-OFF, but QT and QTc dispersions were comparable. The patients' 24-hour heart rates were higher during As2O3-ON than in As2O3-OFF. QTc intervals at each hour were longer during As2O3-ON than in As2O3-OFF. However, QTc prolongation of more than 30 milliseconds only occurred at one time point (2 hours) after oral As2O3, resulting in QTc of more than 500 milliseconds in 3 of 16 patients, all within 4 hours of oral As2O3. Although the standard deviation of normal RR interval was lower during As2O3-ON, ratios of low frequency to high frequency power for As2O3-ON and As2O3-OFF were comparable. No ventricular proarrhythmias were observed. These observations, due to the lower peak plasma arsenic reached during oral As2O3, may explain the relative cardiac safety of oral As2O3. (Blood. 2006;108:103-106)
Introduction
Arsenic trioxide (As2O3) is efficacious in acute promyelocytic leukemia (APL).1-4 It blocks potassium currents IKr and IKs,5 causing QT prolongation. Ventricular tachyarrhythmias were reported in about 30% of patients treated with intravenous As2O36-9 .
As2O3 may be effective in other neoplasms,10 making clinical trials of As2O3 in these diseases pressing. However, reliance on intravenous As2O3 hampers these efforts. Long-term intravenous As2O3 is resource-demanding. Moreover, a potentially fatal cardiac toxicity is worrisome, especially in clinical trials.
We have developed an oral formulation of As2O34,11 . Details of the preparation and pharmacokinetics of oral As2O3 have been previously reported.11 Oral As2O3 gives a similar bioavailability, but lower peak plasma arsenic concentrations, as compared with intravenous As2O311 . Oral As2O3 represents an advance in arsenic therapy. Patients take oral As2O3 at home, rendering long-term therapy feasible, thus facilitating clinical trials. Most importantly, in more than 600 patient weeks of oral As2O3 administered during 5 years, no ventricular tachyarrhythmias were observed.4,11
Prolongation of QT or corrected QT (QTc) increases the risks of ventricular tachyarrhythmias.12,13 QT-interval dispersion measures regional nonhomogeneities of ventricular repolarization.14 Greater dispersions increase ventricular arrhythmias.15 Heart rate variability (HRV) measures the beat-to-beat heart rate variations, correlating with changes in autonomic tone. HRV changes increase cardiac arrhythmias.16
To explain the favorable cardiac side-effect profile of oral As2O3, we studied a cohort of patients on long-term oral As2O3,to determine the cardiac safety and changes of QT intervals and HVR.
Study design
Patients
We studied 17 consecutive patients with relapsed APL (Table 1). All had normal left ventricular ejection fraction and blood biochemistry.
Characteristics and baseline laboratory data of 17 patients with relapsed acute promyelocytic leukemia treated with oral As2O3
Patient no. . | Sex/age, y . | LVEF, % . | Previous treatment . | Creatinine level, μM . | Na level, mM . | K level, mM . | Ca level, mM . | Status* . | Outcome† . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/40 | 55 | ATRA, Dauno | 81 | 141 | 3.7 | 2.41 | CR | CR, 22 mo+ |
| 2 | F/49 | 78 | ATRA, Dauno, VP-16, Ara-C, IDA | 85 | 142 | 3.5 | 2.47 | CR | CR, 85 mo+ |
| 3 | F/35 | 75 | ATRA, Dauno, VP-16, IDA, Ara-C | 59 | 139 | 4.2 | 2.39 | CR | CR, 49 mo+ |
| 4 | F/32 | 65 | ATRA, Dauno, VP-16 | 82 | 141 | 3.8 | 2.46 | CR | CR, 26 mo+ |
| 5 | M/41 | 55 | ATRA, Dauno, Ara-C | 89 | 139 | 2.8 | 2.39 | CR | CR, 55 mo+ |
| 6 | M/76 | 50 | ATRA, Dauno | 146 | 135 | 4.1 | 2.48 | Dead | Sepsis, 15 d |
| 7 | M/46 | 65 | Dauno, VP-16 | 87 | 144 | 3.5 | 2.25 | CR | CR, 26 mo+ |
| 8 | F/55 | 50 | IDA | 87 | 143 | 3.4 | 2.43 | CR | CR, 21 mo+ |
| 9 | M/48 | 50 | ATRA, Dauno, Ara-C, VP-16 | 100 | 140 | 3.6 | 2.38 | CR | CR, 40 mo+ |
| 10 | M/64 | 50 | ATRA, Dauno, Ara-C, IDA | 143 | 141 | 4.3 | 2.27 | CR | CR, 49 mo+ |
| 11 | F/50 | 50 | Dauno, Ara-C, VP-16 | 100 | 137 | 3.9 | 2.30 | CR | CR, 65 mo+ |
| 12 | M/37 | 50 | ATRA, Dauno, Ara-C, VP-16 | 78 | 140 | 3.8 | 2.40 | CR | Dead, R3, 16 mo |
| 13 | M/40 | 65 | ATRA, Dauno, VP-16, Ara-C, IDA | 126 | 135 | 3.7 | 2.33 | CR | CR, 15 mo+ |
| 14 | M/44 | 55 | ATRA, Dauno, Ara-C, VP-16 | 73 | 141 | 3.6 | 2.36 | CR | Dead, TB, 12 mo |
| 15 | M/44 | 50 | ATRA, Dauno, VP-16 | 68 | 142 | 3.6 | 2.38 | CR | CR, 31 mo+ |
| 16 | M/28 | 75 | ATRA, Dauno, VP-16 | 73 | 141 | 3.8 | 2.38 | CR | CR, 11 mo+ |
| 17 | M/31 | 65 | ATRA, Dauno, Ara-C, VP-16 | 88 | 146 | 4.1 | 2.43 | CR | CR, 22 mo+ |
Patient no. . | Sex/age, y . | LVEF, % . | Previous treatment . | Creatinine level, μM . | Na level, mM . | K level, mM . | Ca level, mM . | Status* . | Outcome† . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/40 | 55 | ATRA, Dauno | 81 | 141 | 3.7 | 2.41 | CR | CR, 22 mo+ |
| 2 | F/49 | 78 | ATRA, Dauno, VP-16, Ara-C, IDA | 85 | 142 | 3.5 | 2.47 | CR | CR, 85 mo+ |
| 3 | F/35 | 75 | ATRA, Dauno, VP-16, IDA, Ara-C | 59 | 139 | 4.2 | 2.39 | CR | CR, 49 mo+ |
| 4 | F/32 | 65 | ATRA, Dauno, VP-16 | 82 | 141 | 3.8 | 2.46 | CR | CR, 26 mo+ |
| 5 | M/41 | 55 | ATRA, Dauno, Ara-C | 89 | 139 | 2.8 | 2.39 | CR | CR, 55 mo+ |
| 6 | M/76 | 50 | ATRA, Dauno | 146 | 135 | 4.1 | 2.48 | Dead | Sepsis, 15 d |
| 7 | M/46 | 65 | Dauno, VP-16 | 87 | 144 | 3.5 | 2.25 | CR | CR, 26 mo+ |
| 8 | F/55 | 50 | IDA | 87 | 143 | 3.4 | 2.43 | CR | CR, 21 mo+ |
| 9 | M/48 | 50 | ATRA, Dauno, Ara-C, VP-16 | 100 | 140 | 3.6 | 2.38 | CR | CR, 40 mo+ |
| 10 | M/64 | 50 | ATRA, Dauno, Ara-C, IDA | 143 | 141 | 4.3 | 2.27 | CR | CR, 49 mo+ |
| 11 | F/50 | 50 | Dauno, Ara-C, VP-16 | 100 | 137 | 3.9 | 2.30 | CR | CR, 65 mo+ |
| 12 | M/37 | 50 | ATRA, Dauno, Ara-C, VP-16 | 78 | 140 | 3.8 | 2.40 | CR | Dead, R3, 16 mo |
| 13 | M/40 | 65 | ATRA, Dauno, VP-16, Ara-C, IDA | 126 | 135 | 3.7 | 2.33 | CR | CR, 15 mo+ |
| 14 | M/44 | 55 | ATRA, Dauno, Ara-C, VP-16 | 73 | 141 | 3.6 | 2.36 | CR | Dead, TB, 12 mo |
| 15 | M/44 | 50 | ATRA, Dauno, VP-16 | 68 | 142 | 3.6 | 2.38 | CR | CR, 31 mo+ |
| 16 | M/28 | 75 | ATRA, Dauno, VP-16 | 73 | 141 | 3.8 | 2.38 | CR | CR, 11 mo+ |
| 17 | M/31 | 65 | ATRA, Dauno, Ara-C, VP-16 | 88 | 146 | 4.1 | 2.43 | CR | CR, 22 mo+ |
LVEF indicates left ventricular ejection fraction; F, female; ATRA, all-trans retinoic acid; Dauno, daunorubicin; CR, complete remission; Ara-C, cytosine arabinoside; VP-16, etoposide; IDA, idarubicin; M, male; R3, third relapse; TB, tuberculosis.
Status after completion of the first course of oral As2O3.
Status at latest follow-up, with maintenance therapy comprising oral As2O3 (10 mg/d) and ATRA (45 mg/m2/d), given for 2 weeks every 2 months for a planned 2 years.
Study protocol
Patients received oral As2O3 (10 mg/d) for 2 weeks, followed by a drug-free period of 6 to 8 weeks before the next course. Patients took oral As2O3 at 14:00 every day to match the timing for electrocardiography (ECG) and Holter recording, and blood arsenic assay. Treatment protocol was approved by the institutional review board of Queen Mary Hospital, and all patients gave informed consent in accordance with the Declaration of Helsinki.
ECG and Holter measurement
Data were collected at day 10 of oral As2O3 (As2O3-ON) and 4 weeks after stopping oral As2O3 (As2O3-OFF). Resting 12-lead surface ECGs were recorded (paper-speed: 50 mm/second) for measuring QT intervals. QT intervals at each lead and the corresponding RR-interval at lead II were measured to calculate the QTc (Bazett formula: QTc = QT/square-root of RR interval). QT dispersion was the difference between the maximum and minimal QT measured from each of 12 leads. During As2O3-ON and As2O3-OFF, 24-hour Holter monitoring (Zymed DigiTrak Plus; Zymed 1810, Philips, The Netherlands) was performed to assess circadian QT variations.
Data interpretation
Holter recordings were reviewed and edited manually. Recordings must exceed 20 hours and be of good quality to be analyzed. The time domain (standard deviation of normal RR interval [SDNN]) and frequency domain (ratio of low-frequency to high-frequency power [LF/HF]) of HVR was measured to assess parasympathetic and sympathetic activities, respectively.16 ECG and Holter were analyzed by a cardiologist blinded to patient treatment.
Elemental arsenic levels
After venepuncture, EDTA-anticoagulated blood was immediately separated into plasma and cell fractions. Arsenic levels were measured by inductively coupled plasma mass spectrometry.17
Statistical analysis
Continuous variables were expressed as mean plus or minus standard error of the mean. Comparisons were performed with the Student t test or Fisher exact test (SPSS software, version 10.0). P values less than .05 were considered significant.
Results and discussion
Patients and arsenic levels
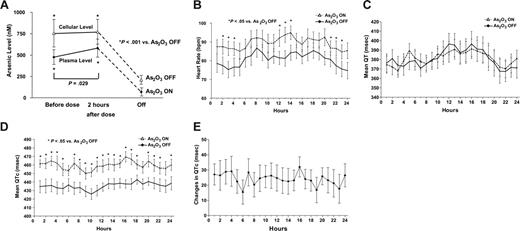
Sixteen patients completed the study (one died of sepsis). Consistently, cellular arsenic levels were significantly higher than plasma arsenic (Figure 1A), similar to previous observations during long-term oral As2O3.13
Changes of arsenic and electrocardiographic parameters during As2O3-ON and As2O3-OFF. (A) Plasma elemental arsenic level. The cellular arsenic level was consistently higher than the plasma arsenic level. At steady state during As2O3-ON, an oral dose of As2O3 (10 mg) did not cause a change in cellular arsenic level. At 4 weeks after cessation of treatment (As2O3-OFF), there was near-complete clearance of arsenic. (B) Circadian variations of heart rate. The heart rate was consistently elevated duringAs2O3-ON. bpm indicates beats per minute. (C) Circadian variations of mean QT intervals. Random samplings of at least 20 templates of 30-second interval recordings (each with > 80% eligible QRS complex) every hour from 24-hour recordings were selected. For each template, mean QT intervals were measured automatically from onset of Q-wave to end of T-wave. Both the pattern and the mean QT intervals were comparable during As2O3-ON and As2O3-OFF. msec indicates milliseconds. (D) Circadian variations of mean QTc. QT intervals were corrected with the mean cycle length of the 30-second interval to calculate QTc. The mean QTc intervals were significantly increased during As2O3-ON. However, the longest mean QTc at 16:00 (2 hours after oral As2O3) was still less than 500 milliseconds. (E) Circadian variation in mean QTc prolongation. The mean QTc prolongation only exceeded 30 milliseconds at one time point (16:00, 2 hours after oral As2O3).
Changes of arsenic and electrocardiographic parameters during As2O3-ON and As2O3-OFF. (A) Plasma elemental arsenic level. The cellular arsenic level was consistently higher than the plasma arsenic level. At steady state during As2O3-ON, an oral dose of As2O3 (10 mg) did not cause a change in cellular arsenic level. At 4 weeks after cessation of treatment (As2O3-OFF), there was near-complete clearance of arsenic. (B) Circadian variations of heart rate. The heart rate was consistently elevated duringAs2O3-ON. bpm indicates beats per minute. (C) Circadian variations of mean QT intervals. Random samplings of at least 20 templates of 30-second interval recordings (each with > 80% eligible QRS complex) every hour from 24-hour recordings were selected. For each template, mean QT intervals were measured automatically from onset of Q-wave to end of T-wave. Both the pattern and the mean QT intervals were comparable during As2O3-ON and As2O3-OFF. msec indicates milliseconds. (D) Circadian variations of mean QTc. QT intervals were corrected with the mean cycle length of the 30-second interval to calculate QTc. The mean QTc intervals were significantly increased during As2O3-ON. However, the longest mean QTc at 16:00 (2 hours after oral As2O3) was still less than 500 milliseconds. (E) Circadian variation in mean QTc prolongation. The mean QTc prolongation only exceeded 30 milliseconds at one time point (16:00, 2 hours after oral As2O3).
ECG and Holter findings
Both QT and QTc were significantly longer during As2O3-ON than in As2O3-OFF (Table 2, P < .01). However, the QT and QTc dispersion were comparable during As2O3-ON and As2O3-OFF (Table 2, P > .05). Holter monitoring showed similar circadian heart rate variations during As2O3-ON and As2O3-OFF, but 24-hour heart rates were consistently higher during As2O3-ON than in As2O3-OFF, being significantly different during late evening (21: 00, 22:00, 23:00), early morning (02:00, 03:00, 04;00), and afternoon (12:00, 13:00, 14:00; P < .05; Figure 1B).
Changes in QT intervals and heart rate variability during oral As2O3 therapy
. | As2O3-ON . | As2O3-OFF . | P . |
|---|---|---|---|
| 12-lead ECG | |||
| QT, ms | 383 ± 8 | 359 ± 8 | .08 |
| QTc, ms | 455 ± 6 | 423 ± 7 | < .01 |
| QT dispersion | 54 ± 4 | 54 ± 4 | .82 |
| QTc dispersion | 65 ± 5 | 62 ± 6 | .78 |
| Holter recording | |||
| SDNN, ms | 90 ± 11 | 118 ± 12 | .02 |
| LF, normalized units | 26 ± 2 | 26 ± 2 | .97 |
| HF, normalized units | 22 ± 3 | 24 ± 3 | .21 |
| LF/HF ratio | 1.36 ± 0.16 | 1.45 ± 0.16 | .30 |
. | As2O3-ON . | As2O3-OFF . | P . |
|---|---|---|---|
| 12-lead ECG | |||
| QT, ms | 383 ± 8 | 359 ± 8 | .08 |
| QTc, ms | 455 ± 6 | 423 ± 7 | < .01 |
| QT dispersion | 54 ± 4 | 54 ± 4 | .82 |
| QTc dispersion | 65 ± 5 | 62 ± 6 | .78 |
| Holter recording | |||
| SDNN, ms | 90 ± 11 | 118 ± 12 | .02 |
| LF, normalized units | 26 ± 2 | 26 ± 2 | .97 |
| HF, normalized units | 22 ± 3 | 24 ± 3 | .21 |
| LF/HF ratio | 1.36 ± 0.16 | 1.45 ± 0.16 | .30 |
For the purposes of this table, P values greater than .05 are not significant.
LF, low frequency; HF indicates high frequency; SDNN, standard deviation of normal to normal RR interval.
QT measurements
QT intervals over 24 hours showed marked variations, although the pattern and measurement at each hour were comparable between As2O3-ON and As2O3-OFF (Figure 1C). Circadian variations of mean QTc intervals (Figure 1D) were comparable between As2O3-ON and As2O3-OFF, but measurements at each hour were significantly longer during As2O3-ON (P < .05, except at 06:00, 19:00, 23:00). The mean differences of QTc between As2O3-ON and As2O3-OFF are shown in Figure 1E. QTc prolongation of more than 30 milliseconds was observed at only one time point (16:00, 2 hours after oral As2O3). No case had QTc prolongation of more than 50 milliseconds. This resulted in a QTc of more than 500 milliseconds in only 3 of 16 patients, all within 4 hours of oral As2O3. Although the SDNN was significantly lower during As2O3-ON than in As2O3-OFF, there was no significantly difference in LF/HF between As2O3-ON and As2O3-OFF (Table 2).
Ventricular arrhythmias
Ventricular premature beats were comparably frequent between As2O3-ON and As2O3-OFF (0.03% ± 0.02% versus 0.03% ± 0.03%; P = .8). No ventricular tachyarrhythmia was observed.
Conclusions and observations
Long-term oral As2O3 significantly increased the mean heart rate and QTc interval, and reduced SDNN. However, QT-interval, QT and QTc dispersions, and LF/HF were not changed. Furthermore, for indicators of proarrhythmic risks,12,13 significant QTc prolongation of more than 30 milliseconds was observed at only a single time point (2 hours after oral As2O3), QTc prolongation never exceeded 50 milliseconds, and a QTc interval of more than 500 milliseconds was observed in only 3 patients within 4 hours of oral As2O3. Importantly, these observations translated into absent ventricular proarrhythmia in all patients. These results were superior to intravenous As2O3, where 26% of patients had QT intervals more than or equal to 500 milliseconds, with QTc interval prolonged by 30 to 60 milliseconds in 36.6% of treatment courses, and by more than 60 milliseconds in 35.4% of patients, resulting in torsades de pointes in 1% of cases.8
The cardiac safety of oral As2O3 may be due to several reasons. First, in ventricular proarrhythmias during intravenous As2O3, As2O3 was infused over 1 to 3 hours.6-9 Pharmacokinetic studies showed that peak plasma arsenic levels after intravenous As2O3 reached 0.34 μM to 2.0 μM.11 However, after oral As2O3, peak arsenic levels were much lower at 0.25 μM to 0.55 μM (although gradual intestinal absorption led to a total area-under-the-curve availability comparable to intravenous As2O311 ). Because of lower peak arsenic levels, QTc prolongations after oral As2O3 were transient and lasted only 4 hours.
Second, arsenic shows a concentration-dependent blockade of IKr and IKs currents (IC50, IKr: 0.14 μM ± 0.01 μM; IKs: 1.13 μM ± 0.06 μM).5 Hence, peak plasma arsenic concentrations reached after intravenous As2O3, but not oral As2O3, are high enough to block IKr and IKs, thereby severely compromising repolarization and increasing susceptibility to ventricular proarrhythmias.
Finally, heart rates were significantly increased during oral As2O3. HVR analysis demonstrated significant decreases in parasympathetic activity without changes in sympathetic activity. The change in sympathetic/parasympathetic balance increases the heart rate, alleviating risks of ventricular tachyarrhythmias during QT prolongation. Hence, despite significant increases in QTc interval, overall QT intervals were unchanged during oral As2O3.
A limitation of this study is absence of an intravenous As2O3 control group. Since formulation of oral As2O3 in 2000, we have not used intravenous As2O3, making it difficult to include an intravenous As2O3 group just for this study. Previous reports of intravenous As2O3 have not measured QT dispersion, HRV, and circadian QT and HR variations, so how they relate to arrhythmias during intravenous As2O3 is difficult to judge. Finally, a 1% frequency of torsades de pointes in intravenous As2O3 might not have been detected in our study size.
Our results provide insights into how an oral formulation improves the cardiac safety of As2O3. Oral As2O3 is convenient and safe for outpatients, and may be the preferred formulation for prolonged arsenic treatment.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2006-01-0054.
The University of Hong Kong holds a provisional patent for oral arsenic trioxide in the treatment of leukemia.
C.-W.S. and W.-Y.A. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the S. K. Yee Medical Foundation for provision of free oral arsenic trioxide to patients.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal