Bacterial infection triggers host inflammation through the activation of immune cells, leading to the elimination of bacteria. However, the regulatory mechanisms of the host inflammatory response remain unknown. Here we report that a subset of potent tolerogenic dendritic cells (DCs), regulatory DCs (DCregs), control the systemic inflammatory response. Unlike normal DCs, which produced proinflammatory cytokines in response to bacterial lipopolysaccharide (LPS), DCregs produced fewer proinflammatory cytokines and instead preferentially produced interleukin-10 (IL-10), and these events involved the expression of IκBNS and Bcl-3 as well as cyclic AMP (cAMP)-mediated activation of protein kinase A (PKA). In addition, DCregs not only suppressed LPS-induced production of proinflammatory cytokines in macrophages, but also reduced their serum levels in mice. Furthermore, DCregs protected mice against the lethality induced by experimental endotoxemia and bacterial peritonitis. The inhibitory effect of DCregs against inflammatory responses involved the production of IL-10. On the other hand, naturally existing tolerogenic DC subsets producing IL-10, CD11clowCD45RBhigh DCs, also suppressed LPS-induced host inflammatory responses. Thus, a subset of tolerogenic DCs act as potential regulators of the host inflammatory response, and they might have preventive and therapeutic potential for the treatment of systemic as well as local inflammatory diseases.
Introduction
Dendritic cells (DCs), the most potent antigen (Ag)-presenting cells (APCs), are defined by their dendritic morphology and unique phenotype and consist of heterogeneous subsets that belong to a myeloid or lymphoid lineage and have differing maturity in both lymphoid and peripheral tissues.1 Immature DCs (iDCs) capture and process Ag in inflammatory tissues, and they develop into mature DCs (mDCs) with up-regulation of major histocompatibility complex (MHC) and costimulatory molecules in inflammatory microenvironments.1 Subsequently, mDCs home into secondary lymphoid tissues, where they present the processed Ags to naive T cells to effectively generate effector T cells.1 In addition, DCs also have the capacity to induce type-1 helper T (TH1) cell and TH2 cell responses, depending on their lineage and activation signals.1 Thereby, DCs play a crucial role in linking between innate and adaptive immunity.1
Recent studies suggest that iDCs play a crucial role in the induction of tolerance through the generation of anergic T cells and regulatory T (TR) cells as well as peripheral deletion of T cells under steady state conditions in vivo.2 Treatment of iDCs with certain immunosuppressive molecules, such as interleukin 10 (IL-10), leads to the generation of tolerogenic DCs that show down-regulation of MHC and costimulatory molecules, and not only show defective T-cell activation but also possess tolerogenic properties.3,4 In addition, several types of murine tolerogenic DCs naturally exist and/or are generated in secondary lymphoid areas under certain conditions.4-6
Host innate immune responses to bacterial infections are mediated primarily by conventional DCs and macrophages.1,7 These cells sense the presence of invading pathogens via various pattern recognition receptors (PRRs) and stimulation of the PRR signaling pathway that initiates the secretion of proinflammatory mediators, which promote host inflammatory responses, resulting in the elimination of microorganisms.7 Despite a good understanding of the process that initiates and promotes host inflammation, little is known about the host immune cells responsible for the inhibition of the inflammatory response.
Sepsis is one representative type of systemic inflammatory response syndrome (SIRS) and is a major cause of morbidity and mortality in neonatal and medical intensive care units.8 Sepsis results from excessive stimulation of the host immune system by lipopolysaccharide (LPS)/endotoxin and other pathogen components to produce various proinflammatory cytokines and chemical mediators, and their overproduction can cause a systemic inflammation that in the worst case produces the pathological outcome of lethal sepsis.8 Despite continuing progress in the development of antibiotics and other supportive care therapies, there is a lack of effective means of prevention of or therapy for sepsis.8,9 Indeed, therapies directed at neutralizing LPS or proinflammatory cytokines can prevent the development of septic shock in animal models, but clinical trials of these therapies have in general failed to improve the outcome of patients with sepsis.8,9 Therefore, there is great enthusiasm about the development of novel strategies for the treatment of sepsis.
To address the mechanism responsible for the control of the host innate immune response, we examined the potential role of DCs in host inflammation using the models of murine experimental endotoxemia and bacterial peritonitis.
Materials and methods
Cell preparation
The preparation of DCs was described previously.10 Briefly, iDCs were prepared by culturing bone marrow (BM) cells (2 × 105/mL) obtained from C57BL/6 mice, BALB/c mice (Charles River Laboratories, Raleigh, NC), or Il10KO mice (Jackson Laboratory, Bar Harbor, ME) with murine granulocyte-macrophage colony-stimulating factor (GM-CSF, 20 ng/mL; Wako Pure Chemical Industries, Osaka, Japan) for 8 days. Regulatory DC precursors (pDCregs) were generated from BM cells (2 × 105/mL) cultured with murine GM-CSF (20 ng/mL), murine IL-10 (20 ng/mL, Wako Pure Chemical Industries), and human transforming growth factor (TGF)-β1 (20 ng/mL, Wako Pure Chemical Industries) for 8 days. Subsequently, iDCs or pDCregs were washed 3 times with cold phosphate buffered saline (PBS) after the generation to prevent carryover of cytokines and were cultured with LPS (1 μg/mL; 055:B5, Sigma, St Louis, MO) for 2 days to generate mDCs or DCregs, respectively.
Preparation of in vitro-generated and splenic CD11clowCD45RBhigh DCs was performed as described in a previous report4 with some modifications. For preparation of in vitro-generated CD11clowCD45RBhigh DCs, BM cells (2 × 105/mL) were cultured with murine GM-CSF (20 ng/mL) and murine IL-10 (20 ng/mL) for 8 days. Subsequently, cells were positively selected with phycoerythrin (PE)-conjugated anti-mouse CD45RB monoclonal antibody (mAb) (BD Biosciences, San Diego, CA) and R-PE Magnetic Particles-DM (BD Biosciences). Based on the phenotype of in vitro-generated CD11clowCD45RBhigh DCs, splenic CD11clowCD45RBhigh DCs were obtained as follows. Splenic DCs were negatively selected from splenocytes using mouse Dendritic Cells Enrichment Set-DM (BD Biosciences). Splenic CD11clowCD45RBhigh DCs were then negatively selected from purified splenic DCs with biotinylated anti-mouse CD11c mAb (BD Biosciences) plus IMag streptavidin Particles Plus-DM (BD Biosciences). The purity of CD11clowCD45RBhigh cells was more than 90%, as indicated by FACS analysis, and fewer than 2 × 105 cells/mouse of these subsets were collected.
For isolation of peritoneal macrophages, mice were given intraperitoneal injections of 1 mL 1% thioglycollate (DIFCO Becton Dickinson, Sparks, MD). Peritoneal exudate cells (PECs) were isolated from the peritoneal cavity 4 days after injection. Macrophages were positively selected from PEC with PE-conjugated anti-mouse CD11b mAb (BD Biosciences) and R-PE Magnetic Particles-DM. The purity of F4/80+CD11b+ cells was more than 90%, as indicated by FACS analysis (data not shown).
CD4+ T cells were negatively selected from spleen mononuclear cells with mouse CD4 T lymphocyte Enrichment Set-DM (BD Biosciences).
Cell stimulation
For stimulation of cells, DCs (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of 8-Br-cyclic AMP (cAMP) (200 μM; Sigma) or Rp-8-Br-cAMPS (1 mM; Calbiochem, San Diego, CA) for 6 or 24 hours in 24-well plates (Becton Dickinson, Franklin Lakes, NJ). In another experiment, macrophages (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of DCs (1.25 × 105 to 5 × 105), control Ig (10 μg/mL; Chemicon International, Temecula, CA), or anti-IL-10 Ab (10 μg/mL; R&D Systems, Minneapolis, MN) for 24 hours. The culture supernatants were collected and stored at -80°C until assayed for cytokines.
Flow cytometry
Cells were stained with fluorescein-conjugated mAbs to mouse CD14, CD40, CD45RB, CD80, CD86, I-A/I-E, or TLR4-MD2 or with isotype-matched control mAb (all from BD Biosciences). Analysis of fluorescence staining was performed with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) and CELLQuest Software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Real-time PCR
Total RNA from DCs (106) was extracted with TRIzol (Life Technologies, Gaithersburg, MD), and cDNA was synthesized with random hexamers as primer using the Superscript kit (Life Technologies). Expression levels of IκBNS11 and Bcl-311 were measured by real-time polymerase chain reaction (PCR) (7300 Real Time PCR System, Applied Biosystems, Foster City, CA) using TaqMan universal PCR Master Mix (Applied Biosystems) and TaqMan probes mix for IκBNS and Bcl-3 (Applied Biosystems) after normalization for the expression of β-actin.
ELISA
Cytokines in culture supernatants or sera were measured with ELISA kits (BioSource International, Camarillo, CA).
Measurement of cAMP concentration
The concentration of intracellular cAMP was determined by enzyme immunoassay (Amersham Biosciences, Piscataway, NJ).
Models for experimental endotoxemia and bacterial peritonitis
We used C57BL/6 mice (5 animals in each group) for murine experimental endotoxemia and bacterial peritonitis.12-14 For LPS-induced endotoxemia, mice were given intraperitoneal injections of a mixture of LPS (1μg/mouse) and D-galactosamine (D-GalN, 10 mg/mouse; Sigma) 2 hours after the intraperitoneal injection of DCs (105 to 106/mouse). Alternatively, mice were given intraperitoneal injections of DCs (106/mouse) 2 hours after intraperitoneal injection of LPS (1 mg/mouse). For bacterial peritonitis, mice were given intraperitoneal injections of DCs (106/mouse) 2 hours after intraperitoneal injection of heat-killed (95°C for 30 minutes) Escherichia coli (DH5α, Invitrogen, Carlsbad, CA; 5 × 108/mouse). For cecal ligation and puncture (CLP), mice were anesthetized with pentobarbital (1 mg/mouse, intraperitoneally), a small abdominal midline incision was made, and the cecum was exposed. The cecum was mobilized and ligated, and punctured through both surfaces once with an 18-gauge needle, and the abdomen was closed. Mice were given intraperitoneal or intravenous injections of DCs (106/mouse) with or without control Ig (1 mg/mouse) or anti-IL-10 Ab (1 mg/mouse) 6 hours after CLP. In another experiment, CLP-treated mice were given intraperitoneal injections of DCs (106/mouse) obtained from Il10KO mice. Survival was monitored at the indicated times for 48 hours (D-GalN-sensitized endotoxemia and bacterial peritonitis) or once daily for 6 days (endotoxemia and CLP). In some experiments, D-GalN-sensitized mice were killed at the indicated time after LPS challenge to obtain blood. The blood was processed to obtain serum and stored at -80°C until it was assayed for cytokines.
Western blot analysis
Cells (5 × 106) were stimulated or not stimulated with LPS (1 μg/mL) for 10 minutes or 60 minutes and lysed with cell lysis buffer (Cell Signaling Technology, Beverly, MA) or NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce, Rockford, IL). The lysates or nuclear extracts were separated on 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Daiichi Pure Chemicals, Tokyo, Japan). After transfer to polyvinylidenefluoride (PVDF) membranes (Millipore, Bedford, MA) and subsequent blocking, the immunoblotting of membranes was performed with a PhosphoPlus p44/42 mitogen-activated protein kinases (MAPKs) (Thr202/Tyr204) antibody kit, a PhosphoPlus p38 MAPK (Thr180/Tyr182) antibody kit, a PhosphoPlus IκB-α (Ser32) antibody kit (all from Cell Signaling Technology) according to the manufacturer's instructions or anti-NF-κB p65 Ab (Santa Cruz Biotechnology, Santa Cruz, CA) plus horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Cell Signaling Technology). Blot was visualized with a LumiGLO system (Cell Signaling Technology).
Detection of thymocyte apoptosis
Thymocytes were prepared from tissue sections from the thymuses of mice 24 hours after CLP. The total number of thymocytes was measured by the trypan blue dye exclusion test, and apoptosis of thymocytes was assessed by flow cytometry using an annexin V-FITC Apoptosis Detection kit (R&D Systems).
T-cell proliferation and cytokine production
CD4+ T cells (105) obtained from normal or CLP-treated mice (24 hours after CLP) were cultured in 96-well plates (Becton Dickinson) with various numbers (1.25 × 103 to 104) of irradiated (15 Gy from a 137Cs source, Gammacell 40 Exactor; MDS Nordion, Kanata, ON, Canada) allogeneic mDCs. [3H]thymidine (Amersham Biosciences, Piscataway, NJ) incorporation was measured on day 3 for the last 18 hours. In another experiment, the culture supernatants were collected and stored at -80°C until being assayed for cytokines.
Statistical analyses
The statistical significance of differences was determined by the Student paired t test or the log-rank test. P values below .01 were considered significant.
Results
DCregs showed defective production of proinflammatory cytokines but produced IL-10
We previously established the methods to obtain murine DCregs, which expressed moderate levels of MHC molecules but low levels of costimulatory molecules as compared with their normal counterparts (Figure 1A) and showed that they are more potent tolerogenic DCs than the previously known tolerogenic DCs with regard to the regulation of T-cell-mediated immune responses through the induction of anergic T cells and TR cells in vivo and in vitro.10
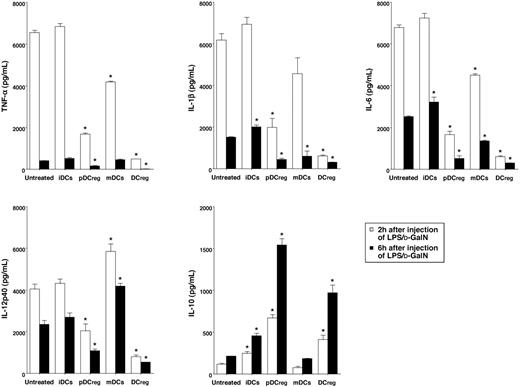
Expression of TLR4-MD2 complex and responsiveness to LPS in DCregs. (A, B) The expression of MHC and costimulatory molecules (A) and the receptors for LPS (B) on DC subsets or macrophages was analyzed by flow cytometry. Data are represented by a histogram in which cells were stained with the indicated mAb (thick lines) or isotype-matched control Ig (thin lines). The results are representative of 4 experiments with similar results. (C) DCs (5 × 105) were stimulated or not stimulated with LPS (1μg/mL) for 6 or 24 hours, and the culture supernatants were analyzed for cytokine production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. *P < .01 compared with iDCs by Student paired t test. (D) Cells (5 × 106) were stimulated or not stimulated with LPS (1μg/mL). Western blot shows the cytoplasmic expression of pErk1/2 and pp38, and pIκB-α before and 10 minutes after LPS stimulation, and the nuclear expression of p65 before and 60 minutes after LPS stimulation. The nonphosphorylated kinases and proteins (ERK1/2, p38, and IκB-α) also were analyzed and serve as the control for protein loading. Representative blots from 3 independent experiments are shown. (E) The expression of IκBNS and Bcl-3 in DCs was measured by real-time PCR. Expression of IκBNS or Bcl-3 was normalized to β-actin, and the data were expressed as comparative fold expression of IκBNS or Bcl-3 compared with iDCs. Representative data from 2 independent experiments are shown. *P < .01 compared with iDCs by Student paired t test. (F) DCs (106) were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours, and the concentration of intracellular cAMP was measured. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 3 experiments with similar results. *P < .01 compared with iDCs by Student paired t test. (G) DCs (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of 8-Br-cAMP (200 μM) or Rp-8-Br-cAMPS (1 mM) for 24 hours, and the culture supernatants were analyzed for IL-10 production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 3 experiments with similar results. *P < .01 compared with LPS stimulation by Student paired t test.
Expression of TLR4-MD2 complex and responsiveness to LPS in DCregs. (A, B) The expression of MHC and costimulatory molecules (A) and the receptors for LPS (B) on DC subsets or macrophages was analyzed by flow cytometry. Data are represented by a histogram in which cells were stained with the indicated mAb (thick lines) or isotype-matched control Ig (thin lines). The results are representative of 4 experiments with similar results. (C) DCs (5 × 105) were stimulated or not stimulated with LPS (1μg/mL) for 6 or 24 hours, and the culture supernatants were analyzed for cytokine production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. *P < .01 compared with iDCs by Student paired t test. (D) Cells (5 × 106) were stimulated or not stimulated with LPS (1μg/mL). Western blot shows the cytoplasmic expression of pErk1/2 and pp38, and pIκB-α before and 10 minutes after LPS stimulation, and the nuclear expression of p65 before and 60 minutes after LPS stimulation. The nonphosphorylated kinases and proteins (ERK1/2, p38, and IκB-α) also were analyzed and serve as the control for protein loading. Representative blots from 3 independent experiments are shown. (E) The expression of IκBNS and Bcl-3 in DCs was measured by real-time PCR. Expression of IκBNS or Bcl-3 was normalized to β-actin, and the data were expressed as comparative fold expression of IκBNS or Bcl-3 compared with iDCs. Representative data from 2 independent experiments are shown. *P < .01 compared with iDCs by Student paired t test. (F) DCs (106) were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours, and the concentration of intracellular cAMP was measured. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 3 experiments with similar results. *P < .01 compared with iDCs by Student paired t test. (G) DCs (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of 8-Br-cAMP (200 μM) or Rp-8-Br-cAMPS (1 mM) for 24 hours, and the culture supernatants were analyzed for IL-10 production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 3 experiments with similar results. *P < .01 compared with LPS stimulation by Student paired t test.
CD14 and/or TLR4-MD2 complex act as receptors for LPS on macrophages and/or DCs.7,15 We therefore examined the expression of TLR4-MD2 complex on pDCregs and DCregs (Figure 1B). Flow cytometric analysis showed that iDCs and pDCregs expressed similar levels of TLR4-MD2 complex, but they did not express CD14. In addition, the expression levels of TLR4-MD2 complex on mDCs and DCregs were reduced as compared with those of iDCs and pDCregs.
We next examined the LPS-induced cytokine production of normal DCs and DCregs (Figure 1C). Stimulation of iDCs with LPS caused vigorous production of TNF-α, IL-1β, IL-6, and IL-12p40, whereas this treatment induced significantly lower production of these proinflammatory cytokines in pDCregs. mDCs constitutively produced large amounts of IL-6 and IL-12p40, but not TNF-α or IL-1β. In addition, mDCs showed slightly lower LPS-induced production of IL-6 and IL-12p40 than iDCs, whereas their production of TNF-α and IL-1β was significantly reduced compared with that of iDCs. In contrast, DCregs exhibited defective production of LPS-induced proinflammatory cytokines. However, pDCregs and DCregs showed higher production of IL-10 than their counterparts.
Stimulation of DCs with LPS caused activation of MAPKs as well as NF-κB, and these events resulted in the functional activation of the DCs.7 To clarify the differences in the activation of the downstream signaling events of TLR4-MD2 complex on normal DCs and DCregs, we examined the phosphorylation of Erk1/2 and p38 (Figure 1D). Stimulation with LPS induced higher levels of phosphorylated Erk1/2 (pErk1/2) and p38 (pp38) in iDCs than mDCs. In addition, the expression levels of both pErk1/2 and pp38 were significantly lower in pDCregs and DCregs than in iDCs and mDCs.
To detect the activation of NF-κB, we examined the expression of phosphorylated IκB-α (pIκB-α) and nuclear translocation of p65 (Figure 1D). iDCs showed higher levels of pIκB-α and nuclear translocation of p65 than mDCs when they were stimulated with LPS. In addition, pDCregs and DCregs showed significantly lower expression level of pIκB-α and nuclear translocation of p65 than their counterparts following stimulation with LPS.
Previous studies have shown that the IL-10-inducible expressions of a member of the IκB protein family, IκBNS and Bcl-3, interfered with the binding of NF-κB p50/p65 to the specific promoters of the targeted proinflammatory cytokines mediated through the interaction with NF-κB p50, and these IκB proteins are required for the IL-10-mediated suppression of proinflammatory cytokines in macrophages.11 We therefore examined the transcriptional expressions of IκBNS and Bcl-3 in normal DCs and DCregs (Figure 1E). Real-time PCR analysis showed that the expression level of IκBNS and Bcl-3 was higher in pDCregs and DCregs than iDCs and mDCs.
Various cAMP-elevating agents have been demonstrated to not only suppress TNF-α synthesis but also enhance IL-10 production in certain cell types.16-18 To clarify the preferential production of IL-10 by pDCregs and DCregs, we measured the endogenous intracellular concentration of cAMP (Figure 1F). mDCs showed slightly higher amount of cAMP than iDCs, and stimulation with LPS had minimal effect. In contrast, both pDCregs and DCregs had increased amounts of cAMP compared witih iDCs and mDCs, and the stimulation with LPS caused further enhancement of the amount of cAMP.
To determine the role of protein kinase A (PKA) on the enhancement of IL-10 synthesis, since PKA is one of the molecular targets of cAMP, we examined the effect of the specific activator (8-Br-cAMP) or inhibitor (Rp-8-Br-cAMPS) of PKA on LPS-induced production of IL-10 in normal DCs and DCregs (Figure 1G). Treatment of these DCs with 8-Br-cAMP increased LPS-induced production of IL-10, whereas Rp-8-Br-cAMPS decreased their production.
DCregs suppressed LPS-induced proinflammatory production in mice with experimental endotoxemia
We tested the effect of DCregs on LPS-induced proinflammatory production in mice with experimental endotoxemia (Figure 2). Intraperitoneal administration of a low dose of LPS (1 μg/mouse) induced transient high production of TNF-α, IL-1β, IL-6, and IL-12p40 in the serum in D-GalN-sensitized mice. A single intraperitoneal injection of iDCs 2 hours before injection of LPS had little or no effect on the serum production of these proinflammatory cytokines, whereas this treatment slightly enhanced the production of serum IL-10. In addition, a single intraperitoneal injection of mDCs 2 hours before injection of LPS slightly reduced the serum production of TNF-α, IL-1β, and IL-6, whereas this treatment enhanced the production of serum IL-12p40. In contrast, a single intraperitoneal injection of DCregs showed more potent suppression of LPS-induced serum proinflammatory cytokine production than injection of pDCregs, whereas a single intraperitoneal injection of pDCregs caused higher production of serum IL-10 than injection of DCregs.
Suppressive effect of DCregs against LPS-induced cytokine production in D-GalN-sensitized mice. D-GalN-sensitized mice were given intraperitoneal injections of LPS (1μg/mouse) 2 hours after the intraperitoneal injection of DCs (106/mouse). Sera were collected at the indicated times after LPS challenge and were analyzed for cytokine production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. *P < .01 compared with untreated control by Student paired t test.
Suppressive effect of DCregs against LPS-induced cytokine production in D-GalN-sensitized mice. D-GalN-sensitized mice were given intraperitoneal injections of LPS (1μg/mouse) 2 hours after the intraperitoneal injection of DCs (106/mouse). Sera were collected at the indicated times after LPS challenge and were analyzed for cytokine production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. *P < .01 compared with untreated control by Student paired t test.
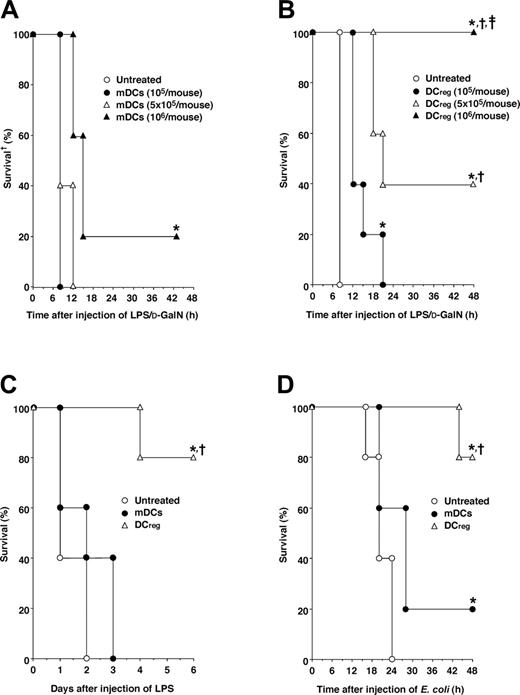
Protective effect of DCregs against lethality induced by murine experimental endotoxemia and bacterial peritonitis. (A, B) D-GalN-sensitized mice (5 animals/group) were given intraperitoneal injections of LPS (1 μg/mouse) 2 hours after the intraperitoneal injection of various doses (105-106/mouse) of mDCs (A) or DCregs (B). *P < .01 compared with the untreated mice by the log-rank test (A, B). †P < .01 compared with mice given injections of 105 DCregs per mouse; ‡, compared with or 5 × 105 DCregs per mouse by the log-rank test (B). (C) Mice (5 animals/group) were given intraperitoneal injections of mDCs or DCregs (106/mouse) 2 hours after intraperitoneal injection of LPS (1 mg/mouse). *P < .01 compared with the untreated mice; †, compared with mice given injections of mDCs, by the log-rank test. (D) Mice (5 animals/group) were given intraperitoneal injections of mDCs or DCregs (106/mouse) 2 hours after intraperitoneal injection of heat-killed E coli (5 × 108/mouse). *P < .01 compared with the untreated mice; †, compared with mice given injections of mDCs, by the log-rank test. Survival was monitored at the indicated times, and the results are representative of 4 experiments with similar results.
Protective effect of DCregs against lethality induced by murine experimental endotoxemia and bacterial peritonitis. (A, B) D-GalN-sensitized mice (5 animals/group) were given intraperitoneal injections of LPS (1 μg/mouse) 2 hours after the intraperitoneal injection of various doses (105-106/mouse) of mDCs (A) or DCregs (B). *P < .01 compared with the untreated mice by the log-rank test (A, B). †P < .01 compared with mice given injections of 105 DCregs per mouse; ‡, compared with or 5 × 105 DCregs per mouse by the log-rank test (B). (C) Mice (5 animals/group) were given intraperitoneal injections of mDCs or DCregs (106/mouse) 2 hours after intraperitoneal injection of LPS (1 mg/mouse). *P < .01 compared with the untreated mice; †, compared with mice given injections of mDCs, by the log-rank test. (D) Mice (5 animals/group) were given intraperitoneal injections of mDCs or DCregs (106/mouse) 2 hours after intraperitoneal injection of heat-killed E coli (5 × 108/mouse). *P < .01 compared with the untreated mice; †, compared with mice given injections of mDCs, by the log-rank test. Survival was monitored at the indicated times, and the results are representative of 4 experiments with similar results.
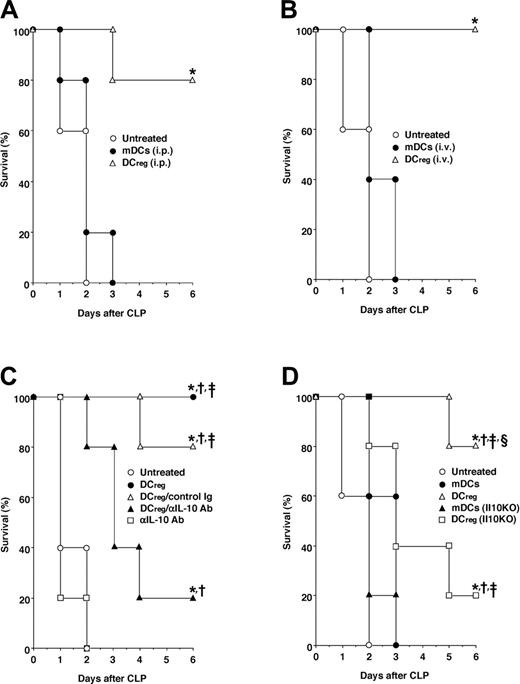
Involvement of IL-10 in the protective effect of DCregs against lethality induced by CLP. Mice (5 animals/group) were given intraperitoneal (A) or intravenous (B) injections of mDCs or DCregs (106/mouse) 6 hours after CLP. *P < .01 compared with the untreated mice by the log-rank test. (C) Mice (5 animals/group) were given intraperitoneal injections of mDCs or DCregs (106/mouse) with or without control Ig (1 μg/mouse) or anti-IL-10 Ab (1 mg/mouse) 6 hours after CLP. *P < .01 compared with the untreated mice, †, compared with mice given injections of anti-IL-10Ab; ‡, compared with mice given injections of DCregs plus anti-IL-10Ab, by the log-rank test. (D) Mice (5 animals/group) given intraperitoneal injections of mDCs or DCregs (106/mouse) obtained from normal mice or Il10KO mice 6 hours after CLP. *P < .01 compared with the untreated mice; †, compared with mice given injections of mDCs; ‡, compared with mice given injections of Il10KO mDCs; or §, compared with mice given injections of Il10KO DCregs, by the log-rank test. Survival was monitored at the indicated times, and the results are representative of 4 experiments with similar results.
Involvement of IL-10 in the protective effect of DCregs against lethality induced by CLP. Mice (5 animals/group) were given intraperitoneal (A) or intravenous (B) injections of mDCs or DCregs (106/mouse) 6 hours after CLP. *P < .01 compared with the untreated mice by the log-rank test. (C) Mice (5 animals/group) were given intraperitoneal injections of mDCs or DCregs (106/mouse) with or without control Ig (1 μg/mouse) or anti-IL-10 Ab (1 mg/mouse) 6 hours after CLP. *P < .01 compared with the untreated mice, †, compared with mice given injections of anti-IL-10Ab; ‡, compared with mice given injections of DCregs plus anti-IL-10Ab, by the log-rank test. (D) Mice (5 animals/group) given intraperitoneal injections of mDCs or DCregs (106/mouse) obtained from normal mice or Il10KO mice 6 hours after CLP. *P < .01 compared with the untreated mice; †, compared with mice given injections of mDCs; ‡, compared with mice given injections of Il10KO mDCs; or §, compared with mice given injections of Il10KO DCregs, by the log-rank test. Survival was monitored at the indicated times, and the results are representative of 4 experiments with similar results.
DCregs protected mice against lethality induced by experimental endotoxemia and bacterial peritonitis
To clarify the regulatory role of DCregs in the inflammatory response in vivo, we examined the effect of DCregs on septic shock induced by experimental endotoxemia and bacterial peritonitis. A single intraperitoneal injection of mDCs 2 hours before injection of a low dose of LPS slightly reduced the lethality in D-GalN-sensitized mice (Figure 3A). In contrast, a single intraperitoneal injection of DCregs 2 hours before the injection of LPS potently prevented D-GalN-sensitized mice from LPS-induced lethality, and the preventive effect occurred in a dose-dependent fashion (Figure 3B). We also observed that DCregs derived from other strains of mice exerted a potent preventive effect against LPS-induced lethality in D-GalN-sensitized mice, whereas mDCs derived from other strains of mice showed a minimal effect (data not shown).
To test the therapeutic effect of DCregs against endotoxemia, mice were administered a high dose of LPS (1 mg/mouse) intraperitoneally and then treated with DCregs, and the survival period was monitored. Treatment with DCregs 2 hours after LPS challenge protected mice from the lethality, whereas treatment with mDCs had no effect (Figure 3C).
We also examined the therapeutic effect of DCregs against lethality caused by bacterial peritonitis (Figure 3D). DCregs showed a more potent protective effect against the lethality induced by bacterial peritonitis than mDCs when mice were given intraperitoneal injections of mDCs or DCregs 2 hours after intraperitoneal injection of heat-killed E coli.
We further evaluated the therapeutic efficacy of DCregs against polymicrobial sepsis induced by CLP, a model that more closely mimics the clinical scenario.11,12 A single intraperitoneal or intravenous injection of DCregs even 6 hours after CLP showed a significant protective effect against CLP-induced lethality, whereas treatment with mDCs had no effect (Figure 4A,B).
These findings indicate that DCregs preferentially produced IL-10 in response to LPS (Figure 1C). We therefore examined the role of IL-10 in the protective effect of DCregs against the lethality induced by CLP. Simultaneous injections of DCregs and anti-IL-10 Ab, but not control Ig, inhibited the protective effect of DCregs against CLP-induced lethality (Figure 4C). Moreover, treatment with DCregs obtained from Il10KO mice19 showed a weaker protective effect against CLP-induced lethality than DCregs obtained from normal mice (Figure 4D).
DCregs suppressed LPS-induced production of proinflammatory cytokines by macrophages mediated through IL-10.
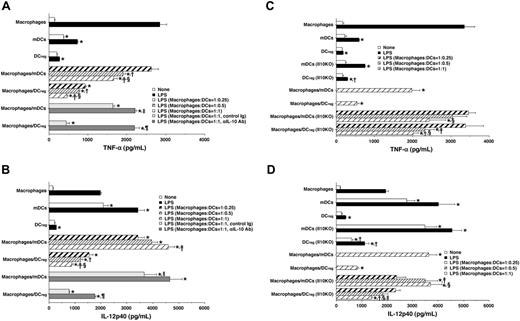
To clarify the mechanism underlying the suppression of inflammatory responses by DCregs, we examined the effect of DCregs on the LPS-induced production of TNF-α and IL-12p40 by macrophages. Stimulation of macrophages with LPS caused higher production of TNF-α than stimulation of mDCs or DCregs, whereas the LPS-induced production of IL-12p40 in mDCs was higher than that in macrophages and DCregs (Figure 5A,B). The addition of mDCs to LPS-stimulated macrophages significantly reduced the production of TNF-α as compared with the production by LPS-stimulated macrophages alone (Figure 5A), whereas their addition additively enhanced the production of IL-12 p40 by LPS-stimulated macrophages (Figure 5B). In contrast, the addition of DCregs to LPS-stimulated macrophages significantly inhibited the production of both TNF-α and IL-12 p40 (Figure 5A,B).
We also examined the role of IL-10 in the DCregs-mediated suppression of LPS-induced production of TNF-α and IL-12p40 by macrophages. Treatment of macrophages and mDCs with anti-IL-10 Ab, but not control Ig, slightly enhanced the LPS-induced production of TNF-α and IL-12p40 (Figure 5A,B). On the other hand, treatment with anti-IL-10 Ab largely abrogated the suppressive effect of DCregs on the production of TNF-α and IL-12p40 by LPS-stimulated macrophages (Figure 5A,B).
To examine whether IL-10 secreted by DCregs plays a direct role in the suppressive effect of these cells, we tested the effect of DCregs obtained from Il10KO mice on the production of TNF-α and IL-12p40 by LPS-stimulated macrophages. mDCs and mDCs obtained from Il10KO mice showed similar ability to produce TNF-α and IL-12p40, whereas DCregs obtained from Il10KO mice produced higher levels of these proinflammatory cytokines in response to LPS than DCregs (Figure 5C,D). Furthermore, DCregs obtained from Il10KO mice showed a weaker suppressive effect against LPS-induced proinflammatory cytokine production by macrophages than DCregs (Figure 5C,D).
Naturally existing tolerogenic DCs suppressed host inflammatory responses
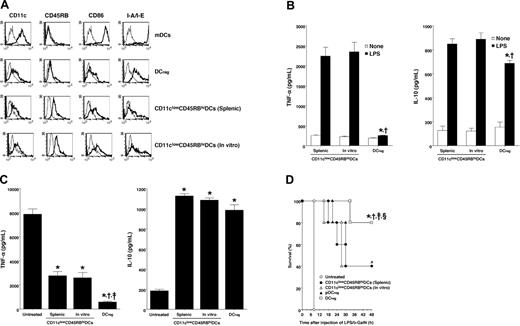
Previous studies have shown that CD11clowCD45RBhigh DCs in the spleen and lymph nodes, which were identical to in vitro-generated IL-10-induced tolerogenic DCs, secreted IL-10 after activation, and these subsets induced Ag-specific tolerance through the differentiation of TR cells.4,6 To address the role of naturally existing tolerogenic DC subsets in host inflammation, we examined the effect of splenic CD11clowCD45RBhigh DCs as well as their in vitro-generated counterparts on LPS-induced inflammatory responses. Flow cytometric analysis showed that splenic and in vitro-generated CD11clowCD45RBhigh DCs exhibited higher expression of CD45RB than DCregs, whereas the expression level of CD11c was similar among these DC subsets (Figure 6A). In addition, these CD11clowCD45RBhigh DCs showed lower expression of I-A/I-E than DCregs, whereas their expression level of CD86 was higher than that of DCregs (Figure 6A). On the other hand, both splenic and in vitro-generated CD11clowCD45RBhigh DCs showed production of IL-10 similar to that of DCregs, whereas these subsets exhibited higher production of TNF-α than DCregs following stimulation with LPS (Figure 6B).
DCregs suppressed LPS-induced production of proinflammatory cytokines by macrophages. (A, B) Macrophages, mDCs, or DCregs (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours. Alternatively, macrophages (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of DCs (1.25 × 105 to 5 × 106), control Ig (10 μg/mL), or anti-IL-10 Ab (10 μg/mL) for 24 hours. The culture supernatants were analyzed for the production of TNF-α (A) or IL-12p40 (B). Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. Statistical analysis was performed by Student paired t test. *P < .01 compared with macrophages; †, compared with cell ratios of 1:0.25 or 1:0.5; ‡, for comparison between cell ratio of 1:0.25 and 1:1; §, for comparison between cell ratios of 1:0.5 and 1:1; ∥, compared with macrophages/mDCs at cell ratios of 1:1; and ¶, P < .01 compared with macrophages/DCregs at cell ratio of 1:1. (C, D) Macrophages, mDCs, or DCregs (5 × 105) obtained from normal mice or Il10KO mice were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours. Alternatively, macrophages (5 × 106) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of DCs (1.25 × 105 to 5 × 106) for 24 hours. The culture supernatants were analyzed for the production of TNF-α (C) or IL-12p40 (D). Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. Statistical analysis was performed by Student paired t test. *P < .01 compared with macrophages; †, for comparison between normal DCs and Il10KO DCs; ‡, for comparison between cell ratios of 1:0.25 and 1:0.5; §, for comparison between cell ratios of 1:0.25 and 1:1; and ∥, for comparison between cell ratios of 1:0.5 and 1:1.
DCregs suppressed LPS-induced production of proinflammatory cytokines by macrophages. (A, B) Macrophages, mDCs, or DCregs (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours. Alternatively, macrophages (5 × 105) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of DCs (1.25 × 105 to 5 × 106), control Ig (10 μg/mL), or anti-IL-10 Ab (10 μg/mL) for 24 hours. The culture supernatants were analyzed for the production of TNF-α (A) or IL-12p40 (B). Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. Statistical analysis was performed by Student paired t test. *P < .01 compared with macrophages; †, compared with cell ratios of 1:0.25 or 1:0.5; ‡, for comparison between cell ratio of 1:0.25 and 1:1; §, for comparison between cell ratios of 1:0.5 and 1:1; ∥, compared with macrophages/mDCs at cell ratios of 1:1; and ¶, P < .01 compared with macrophages/DCregs at cell ratio of 1:1. (C, D) Macrophages, mDCs, or DCregs (5 × 105) obtained from normal mice or Il10KO mice were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours. Alternatively, macrophages (5 × 106) were stimulated or not stimulated with LPS (1 μg/mL) in the presence or absence of DCs (1.25 × 105 to 5 × 106) for 24 hours. The culture supernatants were analyzed for the production of TNF-α (C) or IL-12p40 (D). Data were expressed as mean ± SD of duplicate samples, and the results are representative of 4 experiments with similar results. Statistical analysis was performed by Student paired t test. *P < .01 compared with macrophages; †, for comparison between normal DCs and Il10KO DCs; ‡, for comparison between cell ratios of 1:0.25 and 1:0.5; §, for comparison between cell ratios of 1:0.25 and 1:1; and ∥, for comparison between cell ratios of 1:0.5 and 1:1.
Naturally existing tolerogenic DCs suppressed LPS-induced inflammatory responses. (A) The expression of the indicated cell-surface molecules on DCs was analyzed by flow cytometry. Data are represented by a histogram in which cells were stained with the indicated mAb (thick lines) or isotype-matched control Ig (thin lines). The results are representative of 4 experiments with similar results. (B) DCs (5 × 106) were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours, and the culture supernatants were analyzed for production of TNF-α (left panel) and IL-10 (right panel). *P < .01 compared with splenic CD11clowCD45RBhigh DCs; †, compared with in vitro-generated CD11clowCD45RBhigh DCs by Student paired t test. (C, D) D-GalN-sensitized mice (5 animals/group) were given intraperitoneal injections of LPS (1 μg/mouse) 2 hours after the intraperitoneal injection of splenic CD11clowCD45RBhigh DCs, in vitro-generated CD11clowCD45RBhigh DCs, pDCregs, or DCregs (106/mouse). (C) Sera were collected 2 hours after LPS challenge and were analyzed for cytokine production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 2 experiments with similar results. *P < .01 compared with untreated control; †, compared with splenic CD11clowCD45RBhigh DCs; ‡, compared with in vitro-generated CD11clowCD45RBhigh DCs, by Student paired t test. (D) Survival was monitored at the indicated times, and the results are representative of 2 experiments with similar results. *P < .01 compared with untreated mice; †, compared with mice given injections of splenic CD11clowCD45RBhigh DCs; ‡, compared with mice given injections of in vitro-generated CD11clowCD45RBhigh DCs; §, compared with mice given injections of pDCregs, by the log-rank test.
Naturally existing tolerogenic DCs suppressed LPS-induced inflammatory responses. (A) The expression of the indicated cell-surface molecules on DCs was analyzed by flow cytometry. Data are represented by a histogram in which cells were stained with the indicated mAb (thick lines) or isotype-matched control Ig (thin lines). The results are representative of 4 experiments with similar results. (B) DCs (5 × 106) were stimulated or not stimulated with LPS (1 μg/mL) for 24 hours, and the culture supernatants were analyzed for production of TNF-α (left panel) and IL-10 (right panel). *P < .01 compared with splenic CD11clowCD45RBhigh DCs; †, compared with in vitro-generated CD11clowCD45RBhigh DCs by Student paired t test. (C, D) D-GalN-sensitized mice (5 animals/group) were given intraperitoneal injections of LPS (1 μg/mouse) 2 hours after the intraperitoneal injection of splenic CD11clowCD45RBhigh DCs, in vitro-generated CD11clowCD45RBhigh DCs, pDCregs, or DCregs (106/mouse). (C) Sera were collected 2 hours after LPS challenge and were analyzed for cytokine production. Data were expressed as mean ± SD of duplicate samples, and the results are representative of 2 experiments with similar results. *P < .01 compared with untreated control; †, compared with splenic CD11clowCD45RBhigh DCs; ‡, compared with in vitro-generated CD11clowCD45RBhigh DCs, by Student paired t test. (D) Survival was monitored at the indicated times, and the results are representative of 2 experiments with similar results. *P < .01 compared with untreated mice; †, compared with mice given injections of splenic CD11clowCD45RBhigh DCs; ‡, compared with mice given injections of in vitro-generated CD11clowCD45RBhigh DCs; §, compared with mice given injections of pDCregs, by the log-rank test.
We also compared the inhibitory effect of DCregs and CD11clowCD45RBhigh DCs on LPS-induced cytokine production and lethality in D-GalN-sensitized mice. Both splenic and in vitro-generated CD11clowCD45RBhigh DCs showed lower suppressive effect on LPS-induced serum production of TNF-α than DCregs, whereas similar production of serum IL-10 was observed when both of these types of DCs were injected into mice 2 hours before the injection of LPS (Figure 6C). In addition, treatment with these CD11clowCD45RBhigh DCs showed a preventive effect similar to that of treatment with pDCregs on LPS-induced lethality, although this effect was weaker than that of treatment with DCregs (Figure 6D).
DCregs protected mice from sepsis-induced immunodysregulation in CLP-treated mice
Several studies have shown that increased lymphocyte apoptosis, especially in the thymus, contributes to sepsis-induced lethality, and that blockage of the apoptosis improves the survival of septic mice.12 We therefore examined sepsis-induced thymocyte apoptosis in CLP-treated mice (Figure 7A,B). As reported previously, the significant reduction of thymocytes and marked thymocyte apoptosis were observed in untreated mice 24 hours following CLP. In addition, treatment with DCregs, but not mDCs, ameliorated the reduction of thymocytes and thymocyte apoptosis in CLP-treated mice.
It has been postulated that the systemic inflammatory response caused by sepsis leads to the global immune suppression clinically seen in sepsis syndrome8 ; however, the impact of sepsis on host T-cell responses remains unclear. We therefore examined the ability of CD4+ T cells to proliferate and produce cytokines in CLP-treated mice (Figure 7C,D). CD4+ T cells obtained from untreated mice 24 hours following CLP exhibited marked reduction of the proliferative response as well as of the production of IL-2 and IFN-γ when stimulated with allogeneic mDCs. DCregs, but not mDCs, inhibited the sepsis-induced reduction of the ability of CD4+ T cells to proliferate and produce cytokines.
DCregs protected CLP-treated mice from sepsis-induced immunodysregulation. Mice were given intraperitoneal injections of mDCs or DCregs (106/mouse) 6 hours after CLP. Subsequently, thymocytes and spleen CD4+ T cells were obtained 24 hours after CLP. Thymocytes were obtained from normal mice or CLP-treated mice, and the total number of thymocytes (A) or the incidence of apoptosis was measured by flow cytometry (B). *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. (C,D) CD4+ T cells (105) obtained from normal and CLP-treated mice were cultured with various numbers (1.25 × 103 to 104) of irradiated allogeneic mDCs for 3 days. (C) Proliferative response was measured by [3H]thymidine uptake. *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. (D) Production of IL-2 (left panel) and IFN-γ (right panel) in the culture supernatants was measured. *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. Data were expressed as mean ± SD of triplicate samples, and the results are representative of 4 experiments with similar results.
DCregs protected CLP-treated mice from sepsis-induced immunodysregulation. Mice were given intraperitoneal injections of mDCs or DCregs (106/mouse) 6 hours after CLP. Subsequently, thymocytes and spleen CD4+ T cells were obtained 24 hours after CLP. Thymocytes were obtained from normal mice or CLP-treated mice, and the total number of thymocytes (A) or the incidence of apoptosis was measured by flow cytometry (B). *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. (C,D) CD4+ T cells (105) obtained from normal and CLP-treated mice were cultured with various numbers (1.25 × 103 to 104) of irradiated allogeneic mDCs for 3 days. (C) Proliferative response was measured by [3H]thymidine uptake. *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. (D) Production of IL-2 (left panel) and IFN-γ (right panel) in the culture supernatants was measured. *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. Data were expressed as mean ± SD of triplicate samples, and the results are representative of 4 experiments with similar results.
Discussion
In the present work, we show that a group of tolerogenic DCs are responsible for the regulation of the host inflammatory response that is triggered by bacteria and their products in vivo and in vitro. Our data shed new light on the crucial role of tolerogenic DCs not only in regulating T-cell responses in acquired immunity but also in the damping of host local and systemic inflammatory responses to microbial pathogens in innate immunity.
In contrast to the insights provided by a number of reports about the responses of iDCs to LPS and their functional outcome, the responsiveness of mDCs to LPS remains to be characterized. The ability of mDCs to produce TNF-α and IL-1β in response to LPS was significantly down-regulated compared with that of iDCs. In addition, mDCs exhibited lower activation of MAPKs and NF-κB than iDCs following stimulation with LPS. It is well known that previous exposure of macrophages to LPS induces a hyporesponsive state to secondary stimulation with LPS in terms of proinflammatory cytokine production and that this “LPS tolerance” is associated with the down-regulation of LPS-induced signaling events involving MAPKs and NF-κB.20 Therefore, our results suggest that “LPS tolerance” could be induced in mDCs when they were generated by stimulation of iDCs with LPS. On the other hand, mDCs exhibited constitutively high production of IL-6 and IL-12p40, and they also further responded to stimulation with LPS. These results imply that the maturation-associated changes of the cytokine production profile in conventional DCs might promote an Ag-specific immune response rather than acute inflammation during the bacterial infection.
Although some types of tolerogenic DCs reportedly produced a higher amount of IL-10 instead of the lower production of proinflammatory cytokines compared with normal DCs following certain stimulations,3-6 the molecular regulation of these events remains unclear. We showed that both pDCregs and DCregs produced lower levels of proinflammatory cytokines after stimulation with LPS as compared with iDCs and mDCs, whereas they preferentially produced IL-10. In addition, DCregs obtained from Il10KO mice showed higher production of LPS-induced proinflammatory cytokines than DCregs obtained from normal mice, suggesting that autocrine production of IL-10 by DCregs is partially involved in the defective production by these cells of proinflammatory cytokines. We also showed that pDCregs and DCregs exhibited similar expression of the TLR4-MD2 complex compared with their respective counterparts, whereas the activation of LPS-induced signaling events involving MAPKs and NF-κB were impaired in pDCregs and DCregs. In addition, pDCregs and DCregs showed potent expressions of IκBNS and Bcl-3 when compared with their counterparts, suggesting that these IκB proteins also participate in the suppression of the NF-κB-mediated production of proinflammatory cytokines. Furthermore, pDCregs and DCregs showed constitutively higher amounts of intracellular cAMP than their counterparts, and the blockage of cAMP-mediated activation of PKA dramatically abrogated their LPS-induced production of IL-10. These results suggest that cAMP-mediated activation of PKA is a necessary intermediate event for the preferential production of IL-10 in pDCregs and DCregs. Collectively, both IL-10 and TGF-β1 used in the generation of pDCregs and DCregs appear to specifically regulate the downstream signaling events of the TLR4-MD2 complex leading to the characteristic cytokine production profile.
Treatment of D-GalN-sensitized mice with DCregs completely blocked the LPS-induced increase of the levels of proinflammatory cytokines. DCregs also significantly suppressed the production of TNF-α and IL-12p40 by LPS-stimulated macrophages. In addition, analysis of the suppression with anti-IL-10 Ab and DCregs obtained from Il10KO mice showed that IL-10, especially IL-10 produced by DCregs, is crucially involved in the inhibitory effect of DCregs on the production of these proinflammatory cytokines by LPS-stimulated macrophages. Indeed, treatment of D-GalN-sensitized mice with DCregs obtained from Il10KO mice showed a reduced suppressive effect on the serum production of proinflammatory cytokines (data not shown). Therefore, our results suggest that DCregs suppress the production of the proinflammatory cytokines by inflammatory macrophages through the production of IL-10.
We showed that the treatment of mice with DCregs protected against the lethality induced by experimental endotoxemia and bacterial peritonitis, even when treatment was started after the onset of the disease, possibly through the inhibition of host proinflammatory cytokine production. Previous studies have shown that local production of adenovirus-transduced IL-10 in the thymus and the draining lymph nodes, but not the systemic injection of exogenous IL-10, improved the survival of experimental septic mice, and improvements in survival were associated with the suppression of T-cell apoptosis through the enhancement of Bcl-2 expression and the reduction of caspase-3 activity.21,22 We showed that the blockage of IL-10 reduced the protective effect of DCregs against lethality caused by polymicrobial sepsis in CLP-treated mice. Indeed, treatment of septic mice with DCregs significantly reduced thymocyte apoptosis as well as the suppression of CD4+ T-cell function. We also observed carboxyfluorescein diacetate-succinimidyl ester (CFSE)-labeled DCs in the spleen following intravenous injection into mice.10 In addition, DCregs exhibited higher response to CCL5 but lower response to CCL19 than mDCs, implying that they traffic like semimature DCs (Katsuaki Sato, unpublished data, January 2003). Therefore, our results suggest that the protective effect of DCregs against sepsis may involve the inhibition of macrophage activation in the inflammatory tissues as well as the reduction of the thymocyte apoptosis and CD4+ T-cell functional suppression in secondary lymphoid areas mediated through IL-10. Collectively, our findings suggest that the use of DCregs might have preventive and therapeutic potential for the treatment of sepsis as well as other microbe-mediated systemic and local inflammatory disorders.
Although conventional DCs as well as tissue macrophages actively participate in the elimination of microbial pathogens through the initiation and progression of innate and acquired immunity, the mechanisms responsible for the regulation of host inflammation under pathophysiological conditions have remained largely unknown. We showed that naturally existing tolerogenic DC subsets as well as their in vitro-generated counterparts, which resemble DCregs in terms of preferential production of IL-10, suppressed the inflammatory response in vivo. It has been suggested that “conditioned DC subsets” that exist in mucosal tissues in organized lymphoid organs regulate host immune responses to commensal and pathogenic bacteria.23 In addition, mice devoid of IL-10 expression and function in myeloid-lineage cells develop chronic enterocolitis.19,24 These phenomena lead us to hypothesize that naturally existing tolerogenic DC subsets producing IL-10 exert regulatory activity toward host inflammatory cells, resulting in the suppression of local and systemic inflammatory responses. Further insight into the subset heterogeneity and functional diversity of DCs should contribute to a better understanding of the regulation of innate and acquired immunity under steady-state and pathological conditions.
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-10-4190.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank A. Takeuchi and M. Yamamoto for excellent assistance.


![Figure 7. DCregs protected CLP-treated mice from sepsis-induced immunodysregulation. Mice were given intraperitoneal injections of mDCs or DCregs (106/mouse) 6 hours after CLP. Subsequently, thymocytes and spleen CD4+ T cells were obtained 24 hours after CLP. Thymocytes were obtained from normal mice or CLP-treated mice, and the total number of thymocytes (A) or the incidence of apoptosis was measured by flow cytometry (B). *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. (C,D) CD4+ T cells (105) obtained from normal and CLP-treated mice were cultured with various numbers (1.25 × 103 to 104) of irradiated allogeneic mDCs for 3 days. (C) Proliferative response was measured by [3H]thymidine uptake. *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. (D) Production of IL-2 (left panel) and IFN-γ (right panel) in the culture supernatants was measured. *P < .01 compared with the normal mice; †, compared with untreated mice; ‡, compared with mice given injections of mDCs, by Student paired t test. Data were expressed as mean ± SD of triplicate samples, and the results are representative of 4 experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/9/10.1182_blood-2005-10-4190/2/m_zh80090695110007.jpeg?Expires=1769097330&Signature=udfzMszWilvzp2zanrQSi9278DtH8y-O7~pbwlj3XOmM1puH67XVBr8sM0xBBBwQqnEqnt03zZkYxc7m9uLLzuOL7Io2rFK-xz6YAO9HBF~ARiJz2H39N-4mmTz1ETX01JcGONeO5vi8da7vgbIV1hZgj9w43UzX80y1MC-QzblOWm8yKIPeLSPIF-BLeZPX8wswgFxiAGkGANXzh8omOvb4xptMZrkgPOJSBXzIX1PkhXTNiRn8uC9SHV9RPDkVUbSkFXlu4JiU~B2hnKUtiX77VVM2wtB9pUwFYk32u-eYQRkliUAmsVU-vcgkOnCnJHJjBEEbfCDrhQG-XSmQ1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal