The recent demonstration that platelets express a functional toll-like receptor 4 (TLR4) prompted us to explore the influence of TLR4 polymorphisms (Asp299Gly alone or in combination with Thr399Ile) on thromboxane A2 (TXA2) biosynthesis in vivo. In 17 subjects with TLR4 polymorphisms versus 17 wild type (untreated with aspirin, matched for age, sex, and cardiovascular risk factors), intima-media thickness in the common carotid arteries was significantly lower. Average urinary excretion of 11-dehydro-TXB2, an index of systemic biosynthesis of TX, was significantly reduced by 65%. The urinary excretion of 2,3-dinor-6-keto-prostaglandin F1α, an index of systemic biosynthesis of prostacyclin, was marginally depressed but the prostacyclin/TXA2 biosynthesis ratio was significantly higher than in wild type. Selective inhibition of cyclooxygenase 2-dependent prostacyclin (by rofecoxib or etoricoxib) was associated with increased urinary excretion of 11-dehydro-TXB2 in carriers of TLR4 polymorphisms, but not in wild-type, suggesting a restrainable effect of prostacyclin on platelet function in vivo in this setting. Reduced TXA2 biosynthesis may contribute to the protective cardiovascular phenotype of TLR4 polymorphisms.
Introduction
The toll-like receptor 4 (TLR4) polymorphism Asp299Gly, affecting the extracellular domain of the receptor, is associated with lower concentrations of circulating proinflammatory mediators, which may participate in reduced atherogenesis1 and cardiovascular events2 and with a higher efficacy of statin therapy3 detected in this setting.
The recent demonstration that platelets express a functional TLR44-6 prompted us to explore the influence of TLR4 polymorphisms (Asp299Gly alone or in combination with Thr399Ile) on the biosynthesis in vivo of the prothrombotic and proatherogenic thromboxane A2 (TXA2),7,8 the major product of platelet arachidonic acid metabolism through the activity of cyclooxygenase 1 (COX-1), as compared with wild-type subjects, matched for age, sex, and cardiovascular risk factors, untreated with aspirin. Furthermore, the possible restrainable action of COX-2–dependent prostacyclin on TXA2 biosynthesis in vivo9 was investigated by assessing the effects of selective COX-2 inhibition in this setting.
Study design
Methods
The study protocols were approved by the “G. d'Annunzio” University (Chieti, Italy) Ethics Committee. Written informed consent was obtained from all subjects, according to the Declaration of Helsinki. Of 381 outpatients admitted to our Department of Internal Medicine for a cardiovascular risk program and genotyped for TLR4 polymorphisms Asp299Gly and Thr399Ile,10 24 were carriers of TLR4 polymorphisms (carriage rate: 6.3%). Seven of these subjects were excluded because of concurrent treatment with aspirin. Seventeen subjects were carriers of Asp299Gly allele alone or in combination with Thr399Ile allele (11 heterozygous for the Asp299Gly and Thr399Ile, 5 heterozygous for the Asp299Gly and wild type for the Thr399Ile, 1 heterozygous for Thr399Ile and homozygous for the Asp299Gly). These subjects were compared with 17 subjects with TLR4 wild type, matched for age, sex, and cardiovascular risk factors (Table 1). Subjects did not have any intercurrent infection or inflammatory condition at the time of the assessment and abstained from the use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) for at least 2 weeks before enrollment. The intima-media thickness (IMT) of the common carotid arteries was studied through ultrasonography.1 We assessed plasma soluble (s) CD40L, monocyte chemoattractant protein 1 (MCP-1), and soluble vascular cell adhesion molecule 1(sVCAM-1) by enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN), serum C-reactive protein (CRP) by latex-nephelometry (Dade-Behring, Marburg, Germany), and serum TXB2 levels, a marker of platelet COX-1 activity.11 We collected overnight urine samples (from 8 PM to 8 AM) to assess the levels of 11-dehydro-TXB2 and 2,3-dinor-6-keto-prostaglandin (PG) F1α, major enzymatic metabolites of TXA2 and prostacyclin,12-14 respectively, and 8-iso-PGF2α, an index of oxidative stress in vivo.15
Baseline characteristics of subjects carriers of TLR4 polymorphisms Asp299Gly and Thr399Ile and subjects carriers of TLR4 wild-type
. | TLR4 wild type . | TLR4 polymorphisms . |
|---|---|---|
| Baseline characteristics and values | ||
| No. patients | 17 | 17 |
| Age, y, mean ± SEM | 56 ± 2.7 | 55 ± 2.7 |
| No. women (%) | 8 (47) | 8 (47) |
| Body mass index, kg/m2, mean ± SEM | 30 ± 1.7 | 31 ± 1.5 |
| Hypertension, no. (%) | 13 (76) | 12 (70) |
| Diabetes, no. (%) | 2 (10) | 2 (10) |
| LDL cholesterol level, mM | 3.65 ± 0.3, 3.88 (0.54-6.41) | 3.26 ± 0.7, 3.49 (1.55-4.06) |
| Fibrinogen level, g/L | 3.11 ± 0.19, 2.97 (2.14-4.16) | 3.13 ± 0.15, 3.00 (1.65-4.14) |
| MCP-1 level, pg/mL | 334 ± 22, 310 (206-546) | 350 ± 63, 255 (197-460) |
| sCD40L level, pg/mL | 547 ± 95, 276 (68-1122) | 468 ± 93, 443 (188-1200) |
| CRP level, mg/mL | 0.44 ± 0.07, 0.33 (0.02-0.93) | 0.48 ± 0.07, 0.41 (0.3-1.1) |
| sVCAM level, ng/mL | 697 ± 68, 625 (260-1301) | 465 ± 27, 473 (286-691)* |
| P-selectin level, ng/mL | 96 ± 8.6, 80 (51.6-163) | 79 ± 3.6, 80 (56-119) |
| 8-iso-PGF2α pg/mg creatinine level | 257 ± 39, 188 (89-520) | 279 ± 55, 228 (81-917) |
| IMT, mm | 1.2 ± 0.06, 1.2 (0.7-1.6) | 0.82 ± 0.02, 0.8 (0.7-1)† |
| Medications | ||
| Nitrates, no. (%) | 0 (0) | 1 (5) |
| β-Blockers, no. (%) | 1 (6) | 4 (24) |
| Calcium channel blockers, no. (%) | 4 (24) | 6 (35) |
| ACE inhibitors, no. (%) | 14 (82) | 9 (53) |
| Diuretics, no. (%) | 3 (18) | 5 (29) |
| Statins, no. (%) | 3 (18) | 3 (18) |
. | TLR4 wild type . | TLR4 polymorphisms . |
|---|---|---|
| Baseline characteristics and values | ||
| No. patients | 17 | 17 |
| Age, y, mean ± SEM | 56 ± 2.7 | 55 ± 2.7 |
| No. women (%) | 8 (47) | 8 (47) |
| Body mass index, kg/m2, mean ± SEM | 30 ± 1.7 | 31 ± 1.5 |
| Hypertension, no. (%) | 13 (76) | 12 (70) |
| Diabetes, no. (%) | 2 (10) | 2 (10) |
| LDL cholesterol level, mM | 3.65 ± 0.3, 3.88 (0.54-6.41) | 3.26 ± 0.7, 3.49 (1.55-4.06) |
| Fibrinogen level, g/L | 3.11 ± 0.19, 2.97 (2.14-4.16) | 3.13 ± 0.15, 3.00 (1.65-4.14) |
| MCP-1 level, pg/mL | 334 ± 22, 310 (206-546) | 350 ± 63, 255 (197-460) |
| sCD40L level, pg/mL | 547 ± 95, 276 (68-1122) | 468 ± 93, 443 (188-1200) |
| CRP level, mg/mL | 0.44 ± 0.07, 0.33 (0.02-0.93) | 0.48 ± 0.07, 0.41 (0.3-1.1) |
| sVCAM level, ng/mL | 697 ± 68, 625 (260-1301) | 465 ± 27, 473 (286-691)* |
| P-selectin level, ng/mL | 96 ± 8.6, 80 (51.6-163) | 79 ± 3.6, 80 (56-119) |
| 8-iso-PGF2α pg/mg creatinine level | 257 ± 39, 188 (89-520) | 279 ± 55, 228 (81-917) |
| IMT, mm | 1.2 ± 0.06, 1.2 (0.7-1.6) | 0.82 ± 0.02, 0.8 (0.7-1)† |
| Medications | ||
| Nitrates, no. (%) | 0 (0) | 1 (5) |
| β-Blockers, no. (%) | 1 (6) | 4 (24) |
| Calcium channel blockers, no. (%) | 4 (24) | 6 (35) |
| ACE inhibitors, no. (%) | 14 (82) | 9 (53) |
| Diuretics, no. (%) | 3 (18) | 5 (29) |
| Statins, no. (%) | 3 (18) | 3 (18) |
Values are reported as mean ± SE, median (range).
ACE indicates angiotensin converting enzyme.
P = .001 versus wild-type.
P < .001 versus wild-type.
Six carriers of TLR4 polymorphisms (Asp299Gly alone or in combination with Thr399Ile allele) and 6 wild-type controls were treated with rofecoxib (25 mg daily, at 8 PM, n = 8) or etoricoxib (90 mg daily, at 8 PM, n = 4), 2 highly selective COX-2 inhibitors,16,17 for 5 consecutive days. Etoricoxib was used after the voluntary withdrawal of rofecoxib from the market by Merck.
Statistical analyses
All values were reported as means plus or minus standard error (SE) with median and range values. Comparisons between the 2 groups were made with χ2 test (for discontinuous variables) and the Mann-Whitney test (for eicosanoids and inflammatory biomarkers). The Spearman coefficient(rs) was calculated to quantify correlation between variables. Multivariate linear regression analysis was performed by backward stepping. P values below .05 were assumed to be significant.
In the pharmacologic study, the primary hypothesis was that COX-2 inhibitors would cause a 60% reduction of urinary of 2,3-dinor-6-keto-PGF1α. Assuming an intersubject coefficient of variation (CV) of 22% for urinary excretion of 2,3-dinor-6-keto-PGF1α,18 6 volunteers would allow detecting at least 46% change in its measurement between before and after drug administration with a power of 90% based on 2-tailed tests with P values less than the type I error rate of .05. The secondary response variable was a change in the biosynthesis of TXA2. With this sample size, it was anticipated that this investigation would have 80% power to detect a difference in means of ± 74% in 11-dehydro-TXB2 between before and after drug administration, assuming an intersubject CV of 41%.18 The Wilcoxon matched-pairs test was used to assess whether the treatment affected urinary excretion of 2,3-dinor-6-keto-PGF1α and 11-dehydro-TXB2 from predrug values. The SPSS (SSPS, Chicago, IL) software (version 11.5 for Windows) was used for all statistical analyses.
Results and discussion
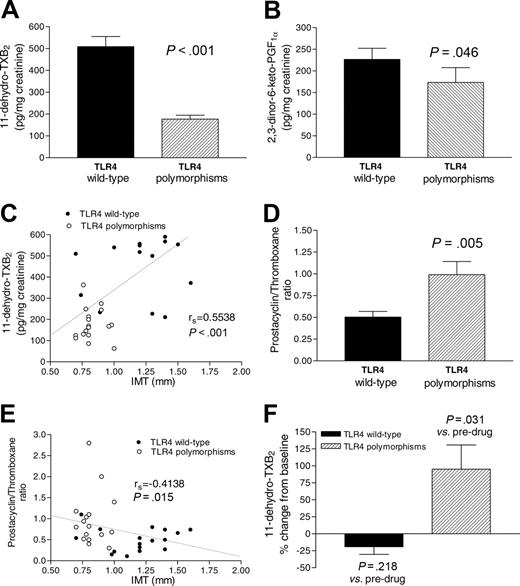
Seventeen subjects with TLR4 polymorphisms, matched with 17 TLR4 wild-type individuals for age, sex, and cardiovascular risk factors (Table 1), presented lower IMT in the common carotid arteries and circulating levels of sVCAM-1 but comparable circulating levels of fibrinogen, sCD40L, MCP-1, P-selectin, and CRP and oxidative stress (Table 1). The urinary excretion of 11-dehydro-TXB2, an index of systemic TXA2 biosynthesis reflecting mainly the contribution of platelet COX-1,19 was profoundly and significantly (P < .001) reduced in carriers of TLR4 polymorphisms versus wild type (Figure 1A; 177 ± 18, 168 [63-363] versus 509 ± 46, 540 [211-836] pg/mg creatinine, respectively, mean ± SE; median [range]). The urinary excretion of 2,3-dinor-6-keto-PGF1α, an index of systemic prostacyclin biosynthesis reflecting mainly the contribution of vascular COX-2,16,20 was only marginally but significantly (P = .046) reduced in carriers of TLR4 polymorphisms (Figure 1B). Bivariate regression analysis showed that urinary 11-dehydro-TXB2 correlated with IMT (rs = 0.5538, P < .001, n = 34; Figure 1C), urinary 2,3-dinor-6-keto-PGF1α (rs = 0.4600, P = .006), sVCAM (rs = 0.4094, P < .01), and P-selectin (rs = 0.3405, P = .04) and inversely with the prostacyclin/TXA2 biosynthesis ratio (rs =–0.6012, P < .001). Using multivariate linear regression analysis, we found that urinary 11-dehydro-TXB2 was independently correlated with Asp299Gly TLR4 allele (coefficient β=–368.8; P = .034) but not with the presence of cardiovascular risk factors. In TLR4 polymorphisms, the production of TXB2 in clotting blood was significantly lower than in wild type (279 ± 22.5, 285 [128-428] versus 391 ± 30, 411 [227-580] ng/mL, P = .018). This small depression of platelet capacity to generate TXA2,11 through COX-1,7 is unlikely to explain by itself the 65% lower biosynthesis of TXA2 in vivo detected in TLR4 polymorphisms versus wild type (Figure 1A). Thus, we presumed that in TLR4 polymorphisms, an endogenous inhibitor might have restrained TXA2 biosynthesis in vivo. The finding of higher prostacyclin/TXA2 biosynthesis ratio detected in carriers of TLR4 polymorphisms than in wild type (Figure 1D) suggested the involvement of COX-2–dependent prostacyclin. Interestingly, IMT inversely correlated with prostacyclin/TXA2 biosynthesis ratio (Figure 1E).
Biosynthesis of TXA2 and prostacyclin. Biosynthesis of TXA2 (A) and prostacyclin in vivo (B), as assessed by the measurement of 11-dehydro-TXB213 and 2,3-dinor-6-keto-PGF1α,14 respectively, in subjects carrying TLR4 polymorphisms and in subjects carrying TLR4 wild type. (C) Correlation between the urinary excretion of 11-dehydro-TXB2 and IMT. (D) Prostacyclin/TXA2 biosynthesis ratio and its correlation with IMT (E). (F) Effects of rofecoxib (25 mg daily) or etoricoxib (90 mg daily) for 5 consecutive days on TXA2 biosynthesis in vivo; overnight urine collections were obtained to evaluate the urinary excretion of 11-dehydro-TXB213 before drug administration and after the last dose of the drugs.
Biosynthesis of TXA2 and prostacyclin. Biosynthesis of TXA2 (A) and prostacyclin in vivo (B), as assessed by the measurement of 11-dehydro-TXB213 and 2,3-dinor-6-keto-PGF1α,14 respectively, in subjects carrying TLR4 polymorphisms and in subjects carrying TLR4 wild type. (C) Correlation between the urinary excretion of 11-dehydro-TXB2 and IMT. (D) Prostacyclin/TXA2 biosynthesis ratio and its correlation with IMT (E). (F) Effects of rofecoxib (25 mg daily) or etoricoxib (90 mg daily) for 5 consecutive days on TXA2 biosynthesis in vivo; overnight urine collections were obtained to evaluate the urinary excretion of 11-dehydro-TXB213 before drug administration and after the last dose of the drugs.
To test this hypothesis, 6 carriers and noncarriers of TLR4 polymorphisms were treated for 5 days with rofecoxib or etoricoxib. Platelet COX-1 activity ex vivo11 was not significantly affected by the 2 drugs both in carriers and noncarriers of TLR4 polymorphisms (–17% ± 11% versus –4% ± 2% of change from predrug values, respectively). A comparable and significant (P = .031) depression of the urinary excretion of 2,3-dinor-6-keto-PGF1α in the 2 groups was found (–59% ± 5% versus –66% ± 6% of change from predrug values, respectively). Predrug values of urinary excretion of 11-dehydro-TXB2 were significantly (P = .026) lower in carriers of TLR4 polymorphisms than in wild type (189 ± 18, 151 [116-350] versus 422 ± 86, 352 [227-826] pg/mg creatinine, respectively). In carriers of TLR4 polymorphisms, selective COX-2 inhibition caused a statistically significant (P = .031) 95% mean increase of urinary excretion of 11-dehydro-TXB2 (338 ± 50, 373 [158-465] pg/mg creatinine) from predrug values (Figure 1F). Differently, in wild type, a nonsignificant (P = .218) 19% mean reduction of TX metabolite excretion (319 ± 44, 298 [155-443] pg/mg creatinine) from predrug values was found (Figure 1F).
Our results are plausible with a restrainable effect of prostacyclin on platelet activation in vivo in carriers of TLR4 polymorphisms. The reason cyclooxygenase 2-dependent prostacyclin failed to constrain TXA2 biosynthesis in vivo in TLR4 wild-type individuals is unclear. It can be speculated that engagement of platelet TLR4 by endogenous ligands (eg, HSP60, minimally modified low-density lipoprotein [LDL], extradomain A of fibronectin)21,22 opposes prostacyclin-dependent cyclic AMP accumulation, which closes exposed binding sites on glycoprotein IIb/IIIa within seconds,23 a mechanism that might be dormant in carriers of TLR4 polymorphisms. Our results pave the way for further studies to elucidate TLR4 downstream signaling in human platelets.
In conclusion, reduced TXA2 biosynthesis may contribute to slackened atherogenesis in carriers of TLR4 polymorphisms. Importantly, the findings of the present study suggest that the cardioprotective phenotype associated with TLR4 polymorphisms may be modified by selective inhibition of COX-2. Such gene-drug interaction might have contributed to the conflicting results of epidemiologic studies on the association between atherosclerosis progression and TLR4 polymorphisms.21,22
Prepublished online as Blood First Edition Paper, January 5, 2006; DOI 10.1182/blood-2005-12-4811.
Supported by grants from the European Union project EICOSANOX (project number LSHM-CT-2004-005033) (P.P.) and MIUR Fondi Ateneo (P.P., E.P. and L.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank the expert editorial assistance of Dr Giovanna Santini.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal