Bortezomib is a novel proteasome inhibitor with significant antimyeloma activity. Its frequent adverse effects are manageable, including gastrointestinal symptoms, peripheral neuropathy, and thrombocytopenia. Severe lung toxicity has not previously been reported. Between June 2004 and September 2005, 13 Japanese patients with multiple myeloma were treated with bortezomib in Toranomon Hospital, Juntendo University School of Medicine, and Jichi Medical School. Four of them developed severe pulmonary complications, and 2 died of respiratory failure without progression of underlying disease. To our knowledge, this is the first report on life-threatening pulmonary adverse effects after bortezomib therapy. Previous clinical studies on bortezomib, mostly in the United States and Europe, have shown low incidences of pulmonary adverse effects. Our study suggests that bortezomib can cause serious lung injury, and that its incidence might vary among different ethnicities. Clinicians need to be alert to the possibility.
Introduction
Bortezomib is a promising agent for multiple myeloma.1-3 Clinical trials for hematologic or solid malignancies are ongoing.4-6 The incidences of serious adverse effects are reportedly less than 5%. Severe pulmonary complications have not been described.1-3 We experienced respiratory failure after bortezomib treatment in Japanese patients. To our knowledge, this is the first report on severe pulmonary complications after bortezomib therapy.
Study design
Between June 2004 and September 2005, 13 Japanese patients (4 men, 9 women) with multiple myeloma at a median age of 54 years (range, 46-79 years) were treated with bortezomib in Toranomon Hospital, Juntendo University School of Medicine, and Jichi Medical School. Four of them developed pulmonary complications. We reviewed all of their medical records, including radiologic and pathologic documents. As of November 2005, bortezomib has not been approved for clinical use in Japan, and was imported by attending physicians at the patients' request. Inclusion to the present report was informed and consent was obtained from the patients or the family members of deceased patients.
Results and discussion
Patient 1
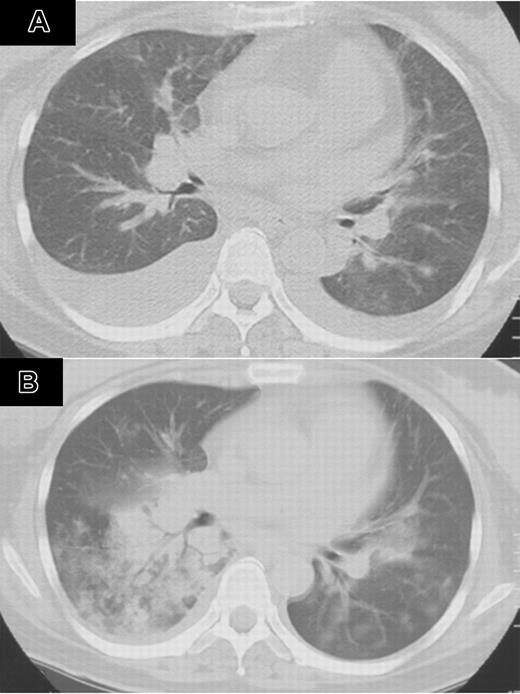
A 47-year-old woman with multiple myeloma, immunoglobulin G (IgG) type, underwent autologous peripheral blood stem cell transplantation (auto-PBSCT) following 200 mg/m2 melphalan. She had no history of smoking. She had a history of invasive pulmonary aspergillosis, which was successfully treated with antifungal agents. Three months after auto-PBSCT, her disease recurred. She had no signs of infection, and her performance status (PS) was 1.7 Bortezomib (1.3 mg/m2) twice a week was given for 2 weeks. Cough and dyspnea developed 8 days after completion of bortezomib. Oxygen saturation dropped to 87%, and chest computed tomography (CT) revealed bilateral pleural effusions (Figure 1A). Peripheral leukocyte count was 5.6 × 109/L. Oxygen and diuretics did not improve her condition. After 40 mg dexamethasone for 4 days, her respiratory symptoms disappeared. The second course of bortezomib was started at the same dose on day 23 of the first course. On day 12 of the second course, she developed recurrent cough, asthmalike symptoms, fever, and dyspnea. Chest CT showed bilateral infiltrates, which rapidly spread to the entire lung fields (Figure 1B). Infection was not identified with sputum cultures. She received granulocyte colony-stimulating factor (G-CSF), beta-lactams, carbapenems, flulconazole, itraconazole, acyclovir, and amphotericin B. Although her respiratory symptoms temporarily improved with 125 to 1000 mg methylprednisolone for 3 days, she died of respiratory failure. Autopsy showed diffuse hyaline membranous changes with partial organization in alveolar cavity and its airway. Pathologic diagnosis was consistent with acute and organizing diffuse alveolar damage.
Patient 2
A 59-year-old woman with multiple myeloma, IgA type, proceeded to auto-PBSCT with a preparative regimen of 200 mg/m2 melphalan. She had no history of smoking. She had a history of pulmonary embolism in November 1999. Six months after auto-PBSCT, her disease recurred and was treated with thalidomide. In June 2005, it became resistant to thalidomide. She had no signs of infection, and her PS was 0.7 She initiated 1.3 mg/m2 bortezomib twice a week for 2 weeks. She developed dyspnea in the morning of the fourth day of completion of bortezomib, when chest CT showed right pleural effusion and pulmonary edema. Peripheral leukocyte count was 7.2 ×109/L. She received trimethoprim/sulfamethoxazole. On the night of the same day, she was intubated for progressive respiratory failure with fever and asthmalike symptoms. Her respiratory failure improved with 1000 mg methylprednisolone for 4 days. As of November 2005, she was alive without respiratory symptoms.
Computed tomography of the chest after treatment with bortezumib (patient 1). (A) CT of the chest 8 days after completion of bortezomib showed bilateral pleural effusion. (B) CT of the chest on day 12 of the second course of bortezomib showed bilateral infiltrates, which rapidly spread to the entire lung field.
Computed tomography of the chest after treatment with bortezumib (patient 1). (A) CT of the chest 8 days after completion of bortezomib showed bilateral pleural effusion. (B) CT of the chest on day 12 of the second course of bortezomib showed bilateral infiltrates, which rapidly spread to the entire lung field.
Patient 3
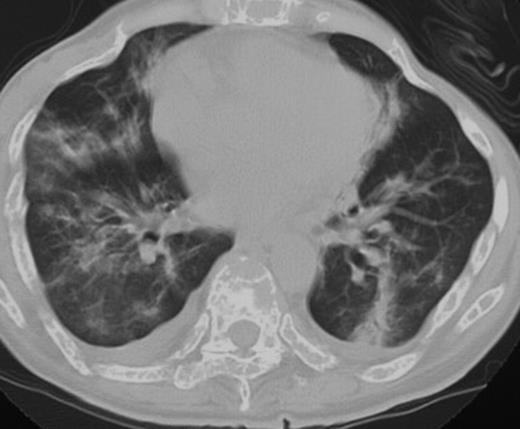
A 48-year-old woman with multiple myeloma, IgD type, underwent unrelated bone marrow transplantation following fludarabine, melphalan, and total-body irradiation in 2002.8 She had no history of smoking or pulmonary problems. The primary disease recurred 3 months after transplantation, and she received 1.3 mg/m2 bortezomib twice a week for 2 weeks. Pretreatment examination failed to show any evidence of infection, and her PS was 1.7 She developed high-grade fever on the day of the fourth dose of bortezomib and severe respiratory failure 5 days after the fourth dose. Chest CT showed bilateral infiltrates with pleural effusions (Figure 2). Peripheral leukocyte count was 4.5 ×109/L (4500/μL), and she had no signs of infection. She received alendronate, fluconazole, allopurinol, trimethoprim/sulfamethoxazole, and famotidine. Her respiratory failure resolved with 500 mg methylprednisolone, and the infiltrates on the chest CT improved. Due to rapid disease progression, she was again treated with bortezomib in combination with 100 mg hydrocortisone with each dose. The disease was well controlled after 11 doses of bortezomib. She never developed respiratory failure.
Patient 4
A 53-year-old woman with multiple myeloma, Bence Jones protein type, underwent auto-PBSCT in 2002. She had no history of smoking or pulmonary problems. The disease recurred in 2003 and was treated with thalidomide and dexamethasone for a year. Due to disease progression in 2004, she proceeded to bortezomib therapy. Pretreatment chest x-ray showed normal findings, and her PS was 1.7 On the next day of 1.3 mg/m2 bortezomib, she developed dyspnea and wheezing with bilateral infiltrates on chest x-ray. Peripheral leukocyte count was 2.1 ×109/L. She developed low-grade fever, although microbiological examination failed to show any evidence of infection. She received fentanyl, elcatonin, and pamidronate. She received 1000 mg methylprednisolone daily. Despite intensive care, she died of respiratory failure 2 days after bortezomib administration. Autopsy was not permitted.
Dicussion
These cases suggest that bortezomib may cause lung injury. The clinical courses were similar in that the patients developed fever and asthmalike symptoms followed by respiratory failure with pulmonary infiltrates on chest CT or x-ray. Negative cultures in these patients indicated that the association of infection was unlikely. Notably, the second course of bortezomib exacerbated respiratory failure in Patient 1. The observation suggests an association between respiratory failure and bortezomib.
The mechanism of lung injury associated with bortezomib is unclear; however, these cases suggest that several types of pathogenesis are involved in the development of lung injury. In Patients 1, 2, and 3, respiratory failure developed after repeated administration of bortezomib, and corticosteroids were effective. Since pharmacokinetics and excretion of bortezomib are not fully elucidated,9,10 and it affects not only nuclear factor (NF)]–κB activity but also various signaling pathways,11,12 bortezomib and/or its metabolites might be accumulated in some patients, causing several cellular reactions associated with pulmonary damages. In Patients 1 and 2, lung injury developed 4 and 8 days after completion of bortezomib, respectively, and responded to corticosteroids. Given that activation of NF-κB leads to inflammatory changes in the lung,13,14 bortezomib withdrawal might have reactivated NF-κB, resulting in lung injury, and the inflammatory reaction may have been controlled with corticosteroids. In Patient 4, respiratory failure developed immediately after the first administration of bortezomib. Rapid disintegration of myeloma cells might have contributed to the pathogenesis of lung damages.
Computed tomography of the chest on the day of the fourth dose of bortezomib showed bilateral pleural effusion and pulmonary infiltrates (patient 3).
Computed tomography of the chest on the day of the fourth dose of bortezomib showed bilateral pleural effusion and pulmonary infiltrates (patient 3).
Previous clinical studies on bortezomib, mostly in the United States and Europe,1-3 have shown low incidences of pulmonary adverse effects. We experienced serious pulmonary complications in 4 of 13 Japanese patients treated with bortezomib. The incidence is higher than those in previous reports.1-3 Although the possibility of different patients' backgrounds in the present and the previous studies cannot be excluded, the history of previous treatments such as auto-PBSCT was not significantly different. Possible genetic predisposition of interstitial pneumonitis has been suggested.15 The incidences of pulmonary complications with gefitinib16 and leflunomide17 have been reported higher in Japanese patients than in those from the United States and Europe; genetic factors might contribute to lung injury by bortezomib. Further investigations are needed to evaluate association between bortezomib toxicities and genetic factors.
In conclusion, bortezomib might cause serious lung injury and its incidence might vary among different ethnicities. Clinicians should be alert to the possibility.
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-11-4541.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Mr Young Sa Lim and Ms Mito Minagawa (RHC USA Corporation, Tokyo, Japan) for helpful advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal