Waldenström macroglobulinemia (WM) is a B-cell disorder characterized by the infiltration of lymphoplasmacytic cells into bone marrow and the presence of an IgM monoclonal gammopathy. As part of the Third International Workshop on WM, held October 7 to 10, 2004 in Paris, France, a consensus panel charged with providing treatment recommendations for WM updated its recommendations on both frontline and salvage therapies. The panel considered encouraging results from recent studies that addressed the use of extended-dose rituximab as well as other treatment options: therapy with either nucleoside analogs and alkylator agents, rituximab in combination with nucleoside analogs, nucleoside analogs plus alkylator agents, or combination chemotherapies, such as CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or cyclophosphamide and dexamethasone. The panel determined that these were reasonable treatment options for WM patients and such therapeutic approaches were likely to yield results that are at least as good as if not better than the currently recommended use of single-agent alkylator, nucleoside analog, or standard-dose rituximab therapy. Such approaches were deemed to be reasonable treatment for WM patients in both the upfront and salvage settings, though randomized studies addressing the efficacy and toxicity of such novel approaches over previously established standard of care options are needed.
Introduction
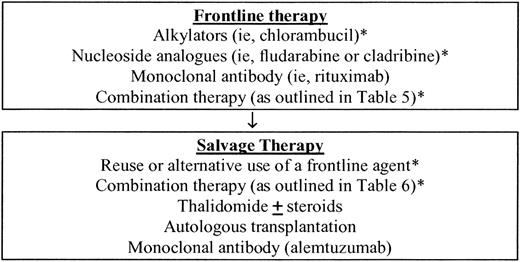
Waldenström macroglobulinemia (WM) is a distinct B-cell lymphoproliferative disorder characterized primarily by the infiltration of lymphoplasmacytic cells into bone marrow and the demonstration of IgM monoclonal gammopathy. This condition is considered to be lymphoplasmacytic lymphoma, as defined by the Revised European American Lymphoma (REAL) and World Health Organization (WHO) classification systems.1 Patients with a disease-related hemoglobin level less than 100 g/L, platelet count less than 100 × 109/L, bulky adenopathy or organomegaly, symptomatic hyperviscosity, severe neuropathy, amyloidosis, cryoglobulinemia, cold-agglutinin disease, or evidence of disease transformation should be considered for therapy.2 Initiation of therapy should not be based on serum monoclonal protein levels per se, and asymptomatic patients should be observed.2 Therapeutic outcomes should be evaluated using updated consensus panel criteria (summarized in “Appendix 1”). As part of the Third International Workshop on WM, which was held October 7 to 10, 2004 in Paris, France, a consensus panel charged with providing treatment recommendations for WM updated its recommendations on both frontline and salvage therapy in view of ongoing clinical trial results (Figure 1). In the original recommendations of Consensus Panel 3, which were formulated at the Second International Workshop on WM, the following therapies were deemed reasonable choices for the first-line therapy of WM: single-agent therapy with alkylating agents, nucleoside analogs, and the monoclonal antibody rituximab.3 In formulating its recommendations, the panel recognized the paucity of randomized clinical trials in WM and concluded that it was not possible to recommend the use of one first-line agent over another. The panel emphasized that individual patient considerations should be weighed in making the choice of a first-line agent, including the presence of cytopenias, need for rapid disease control, age, and candidacy for autologous transplantation therapy. The panel also emphasized that for patients who may be eligible for autologous transplantation, exposure to alkylator agents and nucleoside analogs should be limited in view of reports suggesting depletion of stem cells by these agents.3 The panel also considered options for the treatment of relapsed disease and recommendations for the use of alternate first-line agents, reuse of a first-line agent, use of combination myelotoxic chemotherapy, and the use of thalidomide as a single agent or in combination therapy. Importantly, Consensus Panel 3 affirmed a role for high-dose chemotherapy with autologous peripheral blood cell transplantation in primary refractory or relapsed disease for eligible patients, while stressing that allogeneic or “nonmyeloablative allogeneic” transplantation procedures should be cautiously approached, given the associated high mortality and/or morbidity risks, and should be undertaken in context of a clinical trial.
Updated consensus panel recommendations for frontline and salvage therapy from the Third International Workshop on WM. *The use of alkylator agents and nucleoside analogs should be limited in patients who are eligible for autologous stem cell transplantation.
Updated consensus panel recommendations for frontline and salvage therapy from the Third International Workshop on WM. *The use of alkylator agents and nucleoside analogs should be limited in patients who are eligible for autologous stem cell transplantation.
Clinical trials with rituximab
Since the original recommendations of the Second International Workshop on WM were made 2 years ago, several clinical trials exploring the use of rituximab as well as combination therapy have been completed. Previous studies using standard dose (ie, 4 weekly infusions at 375 mg/m2) of rituximab demonstrated partial responses in approximately 27% of patients,4 a finding affirmed by a recent study from the Eastern Cooperative Group.5 More recent studies have evaluated an extended rituximab dose regimen, wherein patients received rituximab at 375 mg/m twice a week for 4 weeks, repeated at week 12.6,7 The response rates in these studies were higher (44%-48%) than those previously reported with standard doses of rituximab. However, the impact on duration of response for extended over standard-dose therapy remains to be clarified, as does the use of maintenance rituximab in patients with WM.
Time to response after rituximab is slow and exceeds 3 months on the average. In some studies, an inferior response to rituximab was noted when the baseline serum monoclonal protein exceeded 40 g/L or the total IgM level exceeded 6000 mg/dL.6,7 In many patients, a transient increase of serum IgM may occur immediately following initiation of rituximab. Such an increase does not herald treatment failure, and most patients will return to their baseline serum IgM level by 12 weeks.6,8,9 However, patients with baseline serum IgM levels of greater than 50 g/L or serum viscosity of greater than 3.5 centipoise (cp) may be particularly at risk for a hyperviscosity-related event: in these patients, plasmapheresis should be considered in advance of rituximab therapy, with subsequent close serial monitoring of IgM and serum viscosity levels.9 Because of the decreased likelihood of response in patients with higher IgM levels and the possibility that serum IgM and viscosity levels may abruptly rise, rituximab monotherapy should not be used in patients with hyperviscosity symptoms. In one small study, the administration of fludarabine on days 1 to 4 followed by rituximab on day 5 averted a spike in serum IgM levels.10 The sequencing of rituximab following chemotherapy may, therefore, represent a feasible approach to the treatment of WM patients at risk for hyperviscosity. In addition to the baseline serum IgM level, polymorphisms in FcγIIIA (CD16) receptor may also predict responses to rituximab in patients with WM. In a recent analysis of 58 patients with WM who received rituximab, Treon et al11 identified a predictive role for FcγIIIA-158 polymorphisms and response to rituximab. While the results of this study are encouraging, the use of FcγIIIA-158 polymorphisms as a predictor of rituximab response should be considered investigational at this time, pending the outcome of further studies.
Combination therapy with rituximab
Because rituximab is an active and a nonmyelosuppressive agent, its combination with chemotherapy has been explored in WM patients (Table 1). Weber et al12 administered rituximab along with cladribine and cyclophosphamide to 17 previously untreated patients with WM. At least a partial response was documented in 94% of WM patients, including a complete response in 18%. With a median follow-up of 21 months, no patient has relapsed. In a study by the Waldenström's Macroglobulinemia Clinical Trials Group (WMCTG), the combination of rituximab and fludarabine was evaluated in 43 WM patients, 32 (75%) of whom were previously untreated.13 Ninety-one percent of patients demonstrated at least a 25% decrease in serum IgM levels, and response rates were as follows: complete response (CR) 7%; partial response (PR) 74.4%, and minor response (MR) 9.3%. Hematologic toxicity was common, with grade III-IV neutropenia observed in 58% of patients. Two deaths that may have been related to therapy-induced immunosuppression occurred in this study. With a median follow-up of 17 months, 34 (87%) of 39 patients remain in remission. The addition of rituximab to fludarabine and cyclophosphamide has also been explored in the salvage setting by Tam et al,14 wherein 4 of 5 patients demonstrated a response. In another combination study with rituximab, Hensel et al15 administered rituximab along with pentostatin and cyclophosphamide to 17 patients with WM, 9 of who were untreated. Among 11 evaluable patients, a partial response was observed in 90%. In a study by Dimopoulos et al,16 the combination of rituximab, dexamethasone, and cyclophosphamide was used as primary therapy to treat 34 patients with WM. At least a partial response was observed in 78% of patients. Therapy was well tolerated, though one patient died of interstitial pneumonia.
Combination therapy with rituximab in Waldenström macroglobulinemia
Study . | No. patients . | Regimen . | ORR, % . | NR duration, mo . |
|---|---|---|---|---|
| Owen et al1 | 43 | Fludarabine/rituximab | 82 | 17+ |
| Tam et al14 | 5 | Fludarabine/cyclophosphamide/rituximab | 80 | 30+ |
| Weber et al12 | 17 | Cladribine/cyclophosphamide/rituximab | 94 | 21+ |
| Hensel et al15 | 17 | Pentostatin/cyclophosphamide/rituximab | 90 | 12+ |
| Dimoupoulous et al16 | 34 | Dexamethasone/cyclophosphamide/rituximab | 78 | 18+ |
| Hunter et al18 | 13 | CHOP/rituximab | 77 | 9+ |
| Dimopoulous et al6 | 72 | CHOP/rituximab (vs CHOP) | 94 | 48+ |
Study . | No. patients . | Regimen . | ORR, % . | NR duration, mo . |
|---|---|---|---|---|
| Owen et al1 | 43 | Fludarabine/rituximab | 82 | 17+ |
| Tam et al14 | 5 | Fludarabine/cyclophosphamide/rituximab | 80 | 30+ |
| Weber et al12 | 17 | Cladribine/cyclophosphamide/rituximab | 94 | 21+ |
| Hensel et al15 | 17 | Pentostatin/cyclophosphamide/rituximab | 90 | 12+ |
| Dimoupoulous et al16 | 34 | Dexamethasone/cyclophosphamide/rituximab | 78 | 18+ |
| Hunter et al18 | 13 | CHOP/rituximab | 77 | 9+ |
| Dimopoulous et al6 | 72 | CHOP/rituximab (vs CHOP) | 94 | 48+ |
ORR indicates overall response rate; and NR, not reached.
CHOP-R combination therapy
In addition to nucleoside analog–based trials with rituximab, 2 studies have examined CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) in combination with rituximab (CHOP-R). In a randomized frontline study by the German Low Grade Lymphoma Study Group (GLSG) involving 72 patients (71% of who had lymphoplasmacytic lymphoma), a significantly higher response rate (94% vs 69%) was observed among patients receiving CHOP-R versus CHOP, respectively.17 Hunter et al18 have also evaluated CHOP-R in 13 WM patients, 8 and 5 of who were relapsed or refractory to nucleoside analogs and single-agent rituximab, respectively. Among 13 evaluable patients, 10 patients achieved a major response (77%) including 3 CR and 7 PR, and 2 patients achieved a minor response.
Combination therapy with alkylating agents and nucleoside analogs
The addition of alkylating agents to nucleoside analogs has also been explored in WM (Table 2). Weber et al12 administered 2 cycles of oral cyclophosphamide along with subcutaneous cladribine to 37 patients with previously untreated WM. At least a partial response was observed in 84% of patients, and the median duration of response was 36 months. Dimopoulos et al19 examined fludarabine in combination with intravenous cyclophosphamide and observed partial responses in 6 (55%) of 11 WM patients with either primary refractory disease or relapse in treatment. In a recent study involving 49 patients, 35 of who were previously treated, Leblond et al20 evaluated the combination of fludarabine plus cyclophosphamide. Seventy-eight percent of the patients in this study achieved a response, and median time to treatment failure was 27 months. Hematologic toxicity was commonly observed and 3 patients died of treatment-related toxicities. Two interesting findings in this study were the development of acute leukemia in 2 patients, histologic transformation to diffuse large-cell lymphoma in one patient, and 2 cases of solid malignancies (prostate and melanoma), as well as failure to mobilize stem cells in 4 of 6 patients.
Combination therapy with nucleoside analogs and alkylators in Waldenström macroglobulinemia
Study . | No. patients . | Regimen . | ORR, % . | Response duration, mo . |
|---|---|---|---|---|
| Tam et al14 | 9 | Fludarabine/cyclophosphamide | 88 | 13 |
| LeBlond et al20 | 49 | Fludarabine/cyclophosphamide | 78 | 27 |
| Weber et al12 | 37 | Cladribine/cyclophosphamide | 84 | 36 |
| Dimopoulos et al16 | 11 | Dexamethasone/cyclophosphamide/rituximab | 55 | NA |
Study . | No. patients . | Regimen . | ORR, % . | Response duration, mo . |
|---|---|---|---|---|
| Tam et al14 | 9 | Fludarabine/cyclophosphamide | 88 | 13 |
| LeBlond et al20 | 49 | Fludarabine/cyclophosphamide | 78 | 27 |
| Weber et al12 | 37 | Cladribine/cyclophosphamide | 84 | 36 |
| Dimopoulos et al16 | 11 | Dexamethasone/cyclophosphamide/rituximab | 55 | NA |
NA indicates not applicable.
Recommended therapy for WM, in summary
In view of the above data, the consensus panel on therapeutics amended its original recommendations for the therapy of WM3 to include the following as reasonable therapeutic options for the treatment of WM: the use of combination therapy with either nucleoside analogs and alkylator agents, rituximab in combination with nucleoside analogs, nucleoside analogs plus alkylator agents, or combination chemotherapy such as CHOP (Tables 3, 4). The levels of evidence used are those of the US Agency for Health Care Policy and Research21 (summarized in “Appendix 2”). The activity for these combinations was at least on par with if not better than single-agent therapy with either alkylator agents, nucleoside analogs, or rituximab. However, in the absence of randomized clinical trials, it remains to be determined what frontline treatment is optimal and whether efficacy and toxicity of combination therapy is better than single-agent alkylator, nucleoside analog, or rituximab therapy. A randomized study (the WM1 clinical trial) is currently ongoing and is examining the efficacy of alkylator versus nucleoside analogs in patients with WM.22
Frontline therapeutic options for Waldenström macroglobulinemia
Therapeutic class and agents* . | Evidence for efficacy21 . | Level of recommendation21 . |
|---|---|---|
| Alkylator agents | ||
| Chlorambucil | IIa | B |
| Nucleoside analogs | ||
| Cladribine or fludarabine | IIa | B |
| Monoclonal antibody | ||
| Rituximab (standard or extended schedule) | IIa | B |
| Nucleoside analogs plus alkylators | ||
| Cladribine or fludarabine plus cyclophosphamide | IIa | B |
| Nucleoside analogs plus rituximab | ||
| Fludarabine plus rituximab | IIa | B |
| Nucleoside analogs plus alkylators and rituximab | ||
| Cladribine, cyclophosphamide, and rituximab | IIa | B |
| Fludarabine, cyclophosphamide, and rituximab | III | C |
| Pentostatin, cyclophosphamide, and rituximab | III | C |
| Combination chemotherapy plus rituximab | ||
| CHOP and rituximab | IIa | B |
| Cyclophosphamide, dexamethasone, and rituximab | IIa | B |
Therapeutic class and agents* . | Evidence for efficacy21 . | Level of recommendation21 . |
|---|---|---|
| Alkylator agents | ||
| Chlorambucil | IIa | B |
| Nucleoside analogs | ||
| Cladribine or fludarabine | IIa | B |
| Monoclonal antibody | ||
| Rituximab (standard or extended schedule) | IIa | B |
| Nucleoside analogs plus alkylators | ||
| Cladribine or fludarabine plus cyclophosphamide | IIa | B |
| Nucleoside analogs plus rituximab | ||
| Fludarabine plus rituximab | IIa | B |
| Nucleoside analogs plus alkylators and rituximab | ||
| Cladribine, cyclophosphamide, and rituximab | IIa | B |
| Fludarabine, cyclophosphamide, and rituximab | III | C |
| Pentostatin, cyclophosphamide, and rituximab | III | C |
| Combination chemotherapy plus rituximab | ||
| CHOP and rituximab | IIa | B |
| Cyclophosphamide, dexamethasone, and rituximab | IIa | B |
Information has been updated from the original consensus panel recommendations of the Second International Workshop on Waldenström's Macroglobulinemia.4
The choice of appropriate therapy should take into account the candidacy of a patient for high-dose chemotherapy since prolonged use of both alkylating agents and nucleoside analogs can deplete hematopoietic stem cells.
Salvage therapeutic options for Waldenström macroglobulinemia
Therapeutic class and agents . | Evidence for efficacy21 . | Level of recommendation21 . |
|---|---|---|
| Alkylator agents*† | ||
| Chlorambucil | IIa | B |
| Nucleoside analogs*† | ||
| Cladribine or fludarabine | Ib | A |
| Monoclonal antibody† | ||
| Rituximab (standard or extended schedule) | IIa | B |
| Alemtuzumab | III | C |
| Nucleoside analogs plus alkylators*† | ||
| Cladribine or fludarabine plus cyclophosphamide | IIa | B |
| Nucleoside analogs plus rituximab*† | ||
| Fludarabine plus rituximab | IIa | B |
| Nucleoside analogs plus alkylators and rituximab*† | ||
| Cladribine, cyclophosphamide, and rituximab | IIb | B |
| Fludarabine, cyclophosphamide, and rituximab | III | C |
| Pentostatin, cyclophosphamide, and rituximab | III | C |
| Combination chemotherapy plus rituximab | ||
| CHOP and rituximab | III | C |
| Thalidomide | ||
| Thalidomide alone or in combination with dexamethasone | IIa | B |
| Stem cell transplantation‡ | ||
| High-dose chemotherapy and autologous stem cell transplantation | IIa | B |
Therapeutic class and agents . | Evidence for efficacy21 . | Level of recommendation21 . |
|---|---|---|
| Alkylator agents*† | ||
| Chlorambucil | IIa | B |
| Nucleoside analogs*† | ||
| Cladribine or fludarabine | Ib | A |
| Monoclonal antibody† | ||
| Rituximab (standard or extended schedule) | IIa | B |
| Alemtuzumab | III | C |
| Nucleoside analogs plus alkylators*† | ||
| Cladribine or fludarabine plus cyclophosphamide | IIa | B |
| Nucleoside analogs plus rituximab*† | ||
| Fludarabine plus rituximab | IIa | B |
| Nucleoside analogs plus alkylators and rituximab*† | ||
| Cladribine, cyclophosphamide, and rituximab | IIb | B |
| Fludarabine, cyclophosphamide, and rituximab | III | C |
| Pentostatin, cyclophosphamide, and rituximab | III | C |
| Combination chemotherapy plus rituximab | ||
| CHOP and rituximab | III | C |
| Thalidomide | ||
| Thalidomide alone or in combination with dexamethasone | IIa | B |
| Stem cell transplantation‡ | ||
| High-dose chemotherapy and autologous stem cell transplantation | IIa | B |
Information has been updated from the original consensus panel recommendations of the Second International Workshop on Waldenström's Macroglobulinemia.3
The choice of appropriate therapy should take into account the candidacy of a patient for high-dose chemotherapy since prolonged use of both alkylating agents and nucleoside analogs can deplete hematopoietic stem cells.
Reuse of a frontline single agent or combination is reasonable if patient achieved a response duration of at least 1 year; otherwise, use of an alternate single agent or combination is reasonable.
For eligible patients with primary refractory or relapsed disease, high-dose chemotherapy with autologous stem cell transplantation may be reasonable; allogeneic or “nonmyeloablative allogeneic” transplantation procedures should be approached cautiously in view of the associated high mortality and/or morbidity risks and should be undertaken in context of a clinical trial.
Finally, ongoing studies are examining a role for novel therapeutic approaches for WM, including the use of immunomodulating agents with rituximab, alemtuzumab, bortezomib, sildenafil, imatinib mesylate, oblimersen sodium, and nonmyeloablative allogeneic transplants, with encouraging preliminary findings.23-30 The consensus panel on therapeutics reaffirmed its encouragement for the active enrollment of patients with WM on such innovative clinical trials whenever possible.
In summary, encouraging results from recent studies addressing the use of extended-dose rituximab as well as combination therapy for WM suggest that such therapeutic approaches are likely to yield results that are at least as good as if not better than the use of single-agent alkylator, nucleoside analog, or standard-dose rituximab therapy. Such approaches represent reasonable options for the treatment of WM patients in both the upfront as well as salvage settings, though randomized studies addressing the efficacy and toxicity of such novel approaches over previously established options in standards of care are needed to discern such benefits.
Summary of updated response criteria from the 3rd International Workshop on Waldenström's Macroglobulinemia31
Response (abbreviation) . | Criteria . |
|---|---|
| Complete response (CR) | Disappearance of monoclonal protein by immunofixation; no histologic evidence of bone marrow involvement, resolution of any adenopathy/organomegaly (confirmed by CT scan), or signs or symptoms attributable to WM. Reconfirmation of the CR status is required at least 6 weeks apart with a second immunofixation. |
| Partial response (PR) | At least 50% reduction of serum monoclonal IgM concentration on protein electrophoresis and at least 50% decrease in adenopathy/organomegaly on physical examination or on CT scan. No new symptoms or signs of active disease. |
| Minor response (MR) | At least 25% but less than 50% reduction of serum monoclonal IgM by protein electrophoresis. No new symptoms or signs of active disease. |
| Stable disease (SD) | A less-than-25% reduction and less-than-25% increase of serum monoclonal IgM by electrophoresis without progression of adenopathy/organomegaly, cytopenias, or clinically significant symptoms due to disease and/or signs of WM. |
| Progressive disease (PD) | At least 25% increase in serum monoclonal IgM by protein electrophoresis confirmed by a second measurement or progression of clinically significant findings due to disease (ie, anemia, thrombocytopenia, leukopenia, bulky adenopathy/organomegaly) or symptoms (unexplained recurrent fever of at least 38.4°C, drenching night sweats, at least 10% body weight loss, or hyperviscosity, neuropathy, symptomatic cryoglobulinemia, or amyloidosis) attributable to WM. |
Response (abbreviation) . | Criteria . |
|---|---|
| Complete response (CR) | Disappearance of monoclonal protein by immunofixation; no histologic evidence of bone marrow involvement, resolution of any adenopathy/organomegaly (confirmed by CT scan), or signs or symptoms attributable to WM. Reconfirmation of the CR status is required at least 6 weeks apart with a second immunofixation. |
| Partial response (PR) | At least 50% reduction of serum monoclonal IgM concentration on protein electrophoresis and at least 50% decrease in adenopathy/organomegaly on physical examination or on CT scan. No new symptoms or signs of active disease. |
| Minor response (MR) | At least 25% but less than 50% reduction of serum monoclonal IgM by protein electrophoresis. No new symptoms or signs of active disease. |
| Stable disease (SD) | A less-than-25% reduction and less-than-25% increase of serum monoclonal IgM by electrophoresis without progression of adenopathy/organomegaly, cytopenias, or clinically significant symptoms due to disease and/or signs of WM. |
| Progressive disease (PD) | At least 25% increase in serum monoclonal IgM by protein electrophoresis confirmed by a second measurement or progression of clinically significant findings due to disease (ie, anemia, thrombocytopenia, leukopenia, bulky adenopathy/organomegaly) or symptoms (unexplained recurrent fever of at least 38.4°C, drenching night sweats, at least 10% body weight loss, or hyperviscosity, neuropathy, symptomatic cryoglobulinemia, or amyloidosis) attributable to WM. |
US Agency for Health Care Policy and Research: level of evidence
Levels of evidence . | Level type of evidence . | Grades of recommendations . | Recommendation . |
|---|---|---|---|
| Ia | Evidence obtained from meta-analysis of randomized controlled trials. | A | Required: at least one randomized controlled trial as part of the body of literature of overall good quality and consistency addressing specific recommendations. |
| Ib | Evidence obtained from at least one randomized control study. | A | Required: at least one randomized controlled trial as part of the body of literature of overall good quality and consistency addressing specific recommendations. |
| IIa | Evidence obtained from at least one well-designed controlled study without randomization. | B | Required: availability of well-conducted clinical studies but not randomized clinical trials on the topic of recommendation. |
| IIb | Evidence obtained from at least one other kind of well-designed quasi-experimental study. | B | Required: availability of well-conducted clinical studies but not randomized clinical trials on the topic of recommendation. |
| III | Evidence obtained from well-designed, nonexperimental descriptive studies, such as comparative studies, correlation studies, and case-controlled studies. | B | Required: availability of well-conducted clinical studies but not randomized clinicaltrials on the topic of recommendation. |
| IV | Evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. | C | Required: evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. Indicates absence of directly applicable clinical studies of good quality. |
Levels of evidence . | Level type of evidence . | Grades of recommendations . | Recommendation . |
|---|---|---|---|
| Ia | Evidence obtained from meta-analysis of randomized controlled trials. | A | Required: at least one randomized controlled trial as part of the body of literature of overall good quality and consistency addressing specific recommendations. |
| Ib | Evidence obtained from at least one randomized control study. | A | Required: at least one randomized controlled trial as part of the body of literature of overall good quality and consistency addressing specific recommendations. |
| IIa | Evidence obtained from at least one well-designed controlled study without randomization. | B | Required: availability of well-conducted clinical studies but not randomized clinical trials on the topic of recommendation. |
| IIb | Evidence obtained from at least one other kind of well-designed quasi-experimental study. | B | Required: availability of well-conducted clinical studies but not randomized clinical trials on the topic of recommendation. |
| III | Evidence obtained from well-designed, nonexperimental descriptive studies, such as comparative studies, correlation studies, and case-controlled studies. | B | Required: availability of well-conducted clinical studies but not randomized clinicaltrials on the topic of recommendation. |
| IV | Evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. | C | Required: evidence obtained from expert committee reports or opinions and/or clinical experiences of respected authorities. Indicates absence of directly applicable clinical studies of good quality. |
The criteria are summarized from Johnson et al.22
Prepublished online as Blood First Edition Paper, January 12, 2006; DOI 10.1182/blood-2005-02-0833.
Both S.P.T. and D.G.M. received in excess of $10 000 annually from Biogen Idec and Genethon, manufacturers of rituximab.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal