Abstract
Interactions between T cells and antigen-presenting cells (APCs) are the first step in the induction of an adaptive immune response. Here, we show that CD6 and its ligand activated leukocyte cell adhesion molecule (ALCAM) are actively recruited to the antigen-induced dendritic cell (DC)–T-cell contact zone. Moreover, ALCAM-blocking antibodies interfere with DC–T-cell conjugate formation, demonstrating that CD6-ALCAM binding is essential for stable T-cell–APC contact. We now demonstrate that besides their role in establishing initial contacts, CD6-ALCAM interactions are also required during the proliferative phase of the T-cell response; the presence of CD6-blocking antibodies or recombinant ALCAM-Fc proteins results in a strong and sustained inhibition of T-cell proliferation. Furthermore, simultaneous crosslinking of CD6 and CD3 induces enhanced proliferation and transcriptional activity to a similar level as observed after CD3 and CD28 co-crosslinking, demonstrating that CD6 is an important costimulatory molecule. The stability of ALCAM-CD6 binding, which contrasts with transient homotypic ALCAM-ALCAM interactions, further supports the long-lasting effects observed on T-cell proliferation. Taken together, we demonstrate that CD6 and ALCAM form a key adhesive receptor-ligand pair that is not only involved in early DC-T-cell binding but also in sustaining DC-induced T-cell proliferation long after the initial contact has been established.
Introduction
The interaction between a T cell and an antigen-presenting cell (APC) is the central event in the induction of an adaptive immune response and involves different and sequential cellular events. Initially, the T cell transiently adheres to the APC and scans its surface for the presence of specific peptide–major histocompatibility (MHC) complexes in an antigen-independent manner. Several receptor-ligand pairs such as CD2/lymphocyte function-associated antigen-3 (LFA-3), LFA-1/intercellular adhesion molecule-1 (ICAM-1) and ICAM-3, and ICAM-3/dendritic cell (DC)–specific ICAM-3–grabbing nonintegrin (DC-SIGN) have been implicated in these early T-cell–APC interactions.1 In particular, ICAM-1 and -3 play a key role in mediating the initial, antigen-independent adhesion of T cells and APC.1,2 Once the initial contact has been generated, the contact interface is stabilized by molecular reorganization of antigen receptors, adhesion molecules, costimulatory molecules, and the actin cytoskeleton. This highly organized supramolecular structure is known as the immunological synapse.3 Adhesion molecules and T-cell–receptor (TCR)–associated components are segregated into 2 major areas within the immunological synapse: the central supramolecular activation cluster (SMAC), which is enriched in TCR/CD3 complexes, costimulatory molecules (CD4, CD2, CD28), and kinases (protein kinase C-θ [PKC-θ], Lck, and Fyn), and the peripheral SMAC, including LFA-1 and talin.4,5 Synapse formation is accompanied by cytoskeletal rearrangements, induction of tyrosine phosphorylation and an increase in intracellular free Ca2+. Upon Ca2+ mobilization, the nuclear factor of activated T cells (NFAT) is dephosphorylated and translocates to the nucleus, where it acts as a transcriptional regulator of interleukin-2 (IL-2) expression.6
Activated leukocyte cell adhesion molecule (ALCAM; CD166) is a member of the immunoglobulin (Ig) superfamily of proteins.7 Although ALCAM is expressed on a wide variety of cells, within the leukocyte population its expression is particularly high on DC. In addition, monocytic cells in synovium from patients with rheumatoid arthritis show strongly increased ALCAM levels compared with resting monocytes, suggesting that ALCAM is involved in regulating immunologic processes such as inflammation.8 However, the precise role of ALCAM in the immune system is as yet unclear. Similar to several other Ig-like adhesion molecules (NCAM, CEA), ALCAM mediates homotypic ALCAM-ALCAM interactions,7,9,10 but also heterotypic interactions with the T-cell antigen CD6 have been described.7
CD6 is a surface receptor expressed by T lymphocytes, thymocytes, and a subset of B cells.11-13 Its extracellular region contains 3 scavenger receptor cysteine-rich (SRCR) domains, thus including CD6 into the SRCR superfamily of protein receptors.14 To date, ALCAM is the only CD6 ligand identified on immune cells. Although the precise function of CD6 remains to be elucidated, several studies point toward a role for CD6 as a costimulatory molecule in T-cell activation.15-17 CD6 may exert its function via association with CD5, another costimulatory molecule and member of the SRCR superfamily that coprecipitates with CD6 in lymphoid T cells.18 It has been suggested that CD6 fine-tunes CD5 tyrosine phosphorylation by recruiting specific kinases of different families, such as Itk and Lck.19 CD6 physically associates with the TCR/CD3 complex, it relocalizes upon T-cell activation at the central SMAC (cSMAC) and it modulates immunological synapse maturation in a Jurkat-Raji model system.20 Direct evidence showing that CD6 and its ligand ALCAM play a role in mediating contact between resting T lymphocytes and DCs has not been documented. This, and the high expression of ALCAM on monocyte-derived DCs, prompted us to investigate the importance of CD6 and ALCAM in DC–T-cell contact initiation, costimulation, and in the proliferative phase of the T-cell response. Both ALCAM and CD6 are actively recruited at the T-cell–APC interface and contribute to stabilization of the immunological synapse. Moreover, long-term ALCAM-CD6 interactions proved to be crucial for T-cell proliferation and CD6 induces costimulatory signals. We conclude that ALCAM-CD6–mediated adhesion contributes to both early and later stages of DC-induced T-cell activation and proliferation.
Materials and methods
Antibodies and reagents
All chemicals were purchased from Sigma (St Louis, MO) unless stated otherwise. The following antibodies were used: AZN-L50 (anti-ALCAM, mouse IgG2a), J4-81 (anti-ALCAM, mouse IgG1; Antigenix America, Huntington Station, NY), anti–human CD6 (clone M-T605, mouse IgG1; BD Pharmingen, San Diego, CA), anti–human CD28 (clone 28.2, mouse IgG1; BD Pharmingen), NKI-L15 (anti–LFA-1, mouse IgG2a), DF1524 (anti–LFA-1, mouse IgG2b; Sigma), AZN-D1 (anti–DC-SIGN, mouse IgG1), DCN46 (anti–DC-SIGN, mouse IgG2b; BD Pharmingen), and T3b (anti-CD3, mouse IgG2a). Total mouse IgG was from Jackson ImmunoResearch (Westgrove, PA). The following monoclonal antibodies (mAbs) were used to characterize the DCs and peripheral blood lymphocytes (PBLs): anti-CD80, anti-CD86, and FITC-conjugated anti-CD3 (all from BD Pharmingen); anti-CD83, anti-CD20, anti-CD14 (Immunotech; Beckman Coulter, Mijdrecht, the Netherlands); PE-conjugated anti-CD56 (IQ Products, Groningen, the Netherlands), and the isotype controls mouse IgG1, IgG2a, and IgG2b (BD Pharmingen). The following secondary antibodies were used: goat anti–mouse IgG1,IgG2a, or IgG2b conjugated with Alexa-488, Alexa-568, or Alexa-647 (Molecular Probes, Leiden, the Netherlands); FITC-conjugated goat anti–human Fc (Cappel, West Chester, PA), and FITC-conjugated goat anti–mouse (Fab′)2 fragments (Zymed Laboratories, San Francisco, CA).
Recombinant ALCAM-Fc consisting of the extracellular domains of ALCAM fused to the human IgG1 Fc tail was produced and purified as described elsewhere,9 and recombinant CD6-Fc was purchased from R&D Systems (Minneapolis, MN).
Generation of monocyte-derived DCs
Peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats of healthy individuals by Ficoll density centrifugation. Monocytes were isolated from PBMCs by adherence to plastic and were differentiated into immature DCs by culturing for 6 to 7 days in the presence of IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF; 500 and 800 U/mL, respectively; Schering-Plough International, Kenilworth, NJ) in RPMI 1640 medium containing 10% fetal calf serum (FCS). Nonadherent cells (PBLs) were collected for later use. Mature DCs were obtained by culturing immature DCs in the presence of 2 μg/mL lipopolysaccharide (LPS; Sigma) for 24 to 48 hours.
Cell lines, cultures, and expression constructs
All culture media, sera, and antibiotics were purchased from Gibco Invitrogen (Breda, the Netherlands) and were supplemented with 1% antibiotics/antimycotics. Myelomonocytic KG1a cells were cultured in Iscove modified Dulbecco medium containing 10% FCS. Erythroleukemic K562 cells and Jurkat T cells were cultured in RPMI 1640 containing 10% FCS. K562-ALCAM*GFP were generated and maintained as described elsewhere.9 Monomeric red fluorescent protein (RFP) was a gift of Dr T. Jovin (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany). The chimeric CD6-RFP construct was generated by substituting green fluorescent protein (GFP) by RFP in the pEGFP-N1 vector (BD Biosciences Clontech, Palo Alto, CA) using AgeI and NotI restriction sites. CD6 was amplified by polymerase chain reaction (PCR) using the following primers: 5′-ATCAGATCTCACCATGTGGCTCTTCTTC-3′ (forward) and 5′-CGGTGGATCCCGTCCGGCTGCGCTGAT-3′ (reverse). Jurkat T cells were transiently transfected by nucleoporation with an Amaxa Nucleofector (Amaxa, Cologne, Germany) according to the manufacturer's instructions and were cultured for 24 hours in 12-well plates prior to use.
Fluorescent bead adhesion assay
Carboxylate-modified TransFluorSpheres (488/645 nm, 1.0 μM; Molecular Probes, Eugene, OR) were coated with CD6-Fc as described previously for ICAM-3–Fc.21 Cells were resuspended in adhesion buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1 mM CaCl2, 2 mM MgCl2, and 0.5% bovine serum albumin [BSA]) at a final concentration of 5 × 106 cells/mL. Cells (50 000) were preincubated with ALCAM-blocking mAb J4-81 (5 μg/mL) for 10 minutes at room temperature (RT). Ligand-coated fluorescent beads (20 beads/cell) were added, and the suspension was incubated for 30 minutes at 37°C. Adhesion was determined by measuring the percentage of cells that have bound fluorescent beads using flow cytometry.
T-cell proliferation assays
Monocyte-depleted PBLs, isolated from buffy coats of healthy donors, were used in mixed lymphocyte reactions. PBLs (105) of 1 donor were cocultured with 1.7 × 103 DCs of an unrelated donor for 4 to 6 days and proliferation was assessed by [3H]-thymidine (0.037 MBq [1 μCi/well; MP Biomedicals, Irvine, CA) incorporation for 16 hours. Where indicated, antibodies against ALCAM (A2N-L50, 10 μg/mL), CD6 (5 μg/mL), LFA-1 (NKI-L15, 10 μg/mL), or recombinant ALCAM-Fc and CD6-Fc proteins (10 μg/mL) were added at coculture onset. Total mouse IgG and unrelated lymph-node–specific (L)–SIGN–Fc protein were used as negative controls. Alternatively, T cells were stimulated with immobilized anti-CD3 (OKT-3, 0.2 μg/mL), with or without anti-CD28 (4 μg/mL) or anti-CD6 (5 μg/mL), followed by [3H]-thymidine incorporation at day 5.
NFAT reporter gene assay
Round-bottom 96-well plates (Costar, Corning, NY) plates were precoated for 16 hours at 37°C with anti-CD3 (OKT-3, 1 μg/mL) or control mouse IgG in combination with anti-CD28 (4 μg/mL) or anti-CD6 (1 or 5 μg/mL). Jurkat T cells were transfected by Amaxa Nucleofector technology with a pNFAT-Luc construct (containing 4 NFAT binding sites; Stratagene, La Jolla, CA) using 5 μg DNA per 3 × 106 cells. After transfection (24 hours), Jurkat cells were transferred to the precoated plates at 2 × 105 cells per well and were stimulated for 6 hours in RPMI 1640 medium containing 1% FCS at 37°C/5% CO2. Stimulations with PMA (10 ng/mL) and ionomycin (1 μM) served as positive controls. After stimulation, cells were lysed with cell lysis buffer (Promega, Madison, WI), and luciferase activities were measured using chemiluminescent substrates according to the manufacturer's instructions (Promega). Samples were analyzed in a Gen-Probe Leader 50 luminometer (San Diego, CA) and luciferase activities were expressed as the percentage of relative light units (RLUs) of PMA/ionomycin controls.
Conjugate formation analysis
Mature monocyte-derived DCs (105 cells), preincubated for 20 minutes at 37°C with or without 1 μg/mL Staphylococcus enterotoxin B (SEB; Sigma), were mixed with 106 PBLs of an unrelated donor and incubated for 15 or 60 minutes at 37°C with shaking. Where indicated, DCs and PBLs were preincubated for 30 minutes on ice with specific blocking antibodies prior to mixing. Antibodies remained present during cluster formation. The DC-PBL mixture was plated onto poly(L)lysine (50 μg/mL)–coated glass slides and incubated for 5 minutes at RT to let the cells adhere. Next, cells were fixed for 20 minutes at RT with 2% paraformaldehyde in phosphate-buffered saline (PBS). Nonspecific binding sites were blocked with blocking buffer (3% BSA, 10 mM glycine, and 1% human serum in PBS) for 1 hour at RT. Cells were triple stained with anti-ALCAM (AZN-L50, 1 μg/mL), anti-CD6 (5 μg/mL), and anti–LFA-1 (DF1524, 2.5 μg/mL), or anti-CD3 (T3b, 10 μg/mL), anti-CD6 (5 μg/mL) and anti–DC-SIGN (DCN46, 10 μg/mL) in blocking buffer for 1 hour at RT, followed by incubation with Alexa Fluor 568–conjugated GαM-IgG2a, Alexa Fluor 488–conjugated GαM-IgG1 and Alexa Fluor 647–conjugated GαM-IgG2b for an additional 1 hour. DC–T-cell conjugates were analyzed by confocal laser scanning microscopy at 488 nm with a krypton/argon laser on an MRC1024 confocal microscope (BioRad, Hercules, CA) with a 60 ×/1.4 numeric aperture (NA) oil objective lens.
Time-lapse fluorescence confocal microscopy
Jurkat-CD6*RFP cells (4 × 105) in RPMI 1640 without phenol red (Gibco Invitrogen) were allowed to adhere onto fibronectin (20 μg/mL)–coated glass slides for 10 minutes at 37°C. K562-ALCAM*GFP cells (2 × 105) were added to the adherent Jurkat cells, and cells were analyzed at 37°C with a Zeiss LSM 510 microscope equipped with a type S heated stage CO2 controller and PlanApochromatic 63 ×/1.4 NA oil immersion differential interference contrast (DIC) lens (Carl Zeiss, Jena, Germany). Images were taken at 20-second intervals for 60 minutes. Cells were imaged using Zeiss LSM Image Browser version 3.2 (Carl Zeiss) and processed with Image J version 1.32j software (National Institutes of Health; http://rsb.info.nih.gov/ij).
Results
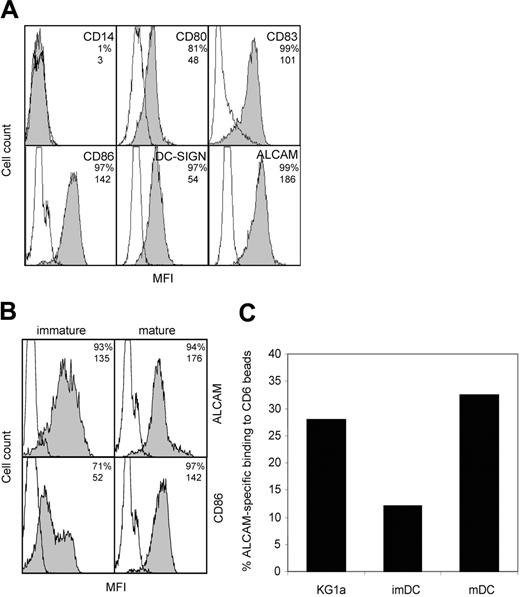
ALCAM is highly expressed on monocyte-derived DCs and mediates adhesion to CD6
ALCAM-CD6 interactions are implicated in the formation of DC–T-cell contacts. We investigated the expression levels of ALCAM on monocyte-derived DCs, relative to a panel of known DC markers. Mature DCs express ALCAM at levels comparable to maturation marker CD86 (Figure 1A). Upon maturation, ALCAM expression remains unchanged, while CD86 expression is significantly enhanced (Figure 1B). To investigate whether ALCAM expression is related to DC function or is induced by in vitro culturing, we analyzed ALCAM expression on peripheral blood DCs. Freshly isolated CD11c+ blood DCs show ALCAM expression that is maintained upon maturation (Figure S1; see the Supplemental Materials link at the top of the online article on the Blood website). Immature and mature monocyte-derived DCs express similar levels of ALCAM (Figure 1A) and are capable of binding to CD6-Fc coated beads (Figure 1C). However, mature DCs show a markedly higher percentage of ALCAM-specific adhesion, comparable with that observed with the control myelomonocytic cell line KG1a that expresses high levels of ALCAM. The difference in CD6-Fc beads binding between immature and mature DCs may be due to a different distribution of ALCAM molecules during DC development, leading to altered ligand-binding avidities.
ALCAM is expressed by monocyte-derived DCs and mediates adhesion to CD6. (A) Characterization of mature DCs. DCs were stained with mAbs against costimulatory molecules and DC-specific molecules. Empty histograms represent isotype-matched controls; shaded histograms, antibody staining. Percentage of positive cells and mean fluorescence intensity (MFI) values are indicated in the top right corner of each subpanel. (B) ALCAM expression is not altered upon maturation of DCs. Immature DCs (left) were stimulated with LPS (2 μg/mL) for 2 days to obtain mature DCs (right). Open histograms represent isotype-matched controls; shaded histograms, antibody staining. Percentage of positive cells and MFI values are indicated in the top right corner of each subpanel. (C) DCs adhere to CD6-Fc–coated microbeads. Immature DCs, mature DCs, and control myelomonocytic KG1a cells were allowed to adhere to CD6-Fc–coated fluorescent beads after preincubation with or without mAb J4-81 (anti-ALCAM) for 45 minutes at 37°C and were analyzed by flow cytometry. ALCAM-specific adhesion is expressed as the adhesion percentage after subtraction of adhesion in the presence of mAb J4-81. One representative experiment of 3 is shown.
ALCAM is expressed by monocyte-derived DCs and mediates adhesion to CD6. (A) Characterization of mature DCs. DCs were stained with mAbs against costimulatory molecules and DC-specific molecules. Empty histograms represent isotype-matched controls; shaded histograms, antibody staining. Percentage of positive cells and mean fluorescence intensity (MFI) values are indicated in the top right corner of each subpanel. (B) ALCAM expression is not altered upon maturation of DCs. Immature DCs (left) were stimulated with LPS (2 μg/mL) for 2 days to obtain mature DCs (right). Open histograms represent isotype-matched controls; shaded histograms, antibody staining. Percentage of positive cells and MFI values are indicated in the top right corner of each subpanel. (C) DCs adhere to CD6-Fc–coated microbeads. Immature DCs, mature DCs, and control myelomonocytic KG1a cells were allowed to adhere to CD6-Fc–coated fluorescent beads after preincubation with or without mAb J4-81 (anti-ALCAM) for 45 minutes at 37°C and were analyzed by flow cytometry. ALCAM-specific adhesion is expressed as the adhesion percentage after subtraction of adhesion in the presence of mAb J4-81. One representative experiment of 3 is shown.
ALCAM-CD6 interactions are essential to promote proliferation of resting T cells induced by mature DCs
We analyzed the role of ALCAM and CD6 in T-cell activation and proliferation induced by allogeneic mature DCs and used freshly isolated total human PBLs as a T-cell source. CD6 and ALCAM expression on these cells was measured by flow cytometry (Figure 2A). As expected, CD6 expression was detected on the majority of CD3+ T cells. Interestingly, NK (CD3–CD56+) cells, but not peripheral B (CD3–CD20+) cells, also expressed substantial levels of CD6. In agreement with previous findings,17 resting T cells did not express ALCAM. We detected low levels of ALCAM on B cells but not on NK cells.
To demonstrate the relevance of ALCAM-CD6 interactions for T-cell activation, DCs and PBLs from different donors were cocultured in the presence or absence of specific blocking antibodies as well as recombinant soluble ALCAM-Fc and CD6-Fc molecules that were added at the onset of the cocultures. None of the used antibodies induced toxicity since both trypan blue (not shown) and propidium iodide stainings (Figure S2) demonstrated that most (> 85%) PBLs were viable after 5 days of coculture in the presence of the indicated antibodies.
ALCAM-CD6 interactions are essential for DC-induced T-cell proliferation. (A) Human PBLs were triple-stained and gated for T cells, NK cells, and B cells. CD6 and ALCAM staining is represented by shaded histograms; isotype-matched controls are represented by open histograms. One representative experiment of 3 is shown. Percentage of positive cells and MFI values are indicated in the top right corner of each subpanel. (B) PBLs (105) were stimulated in vitro with 1.7 × 103 mature monocyte-derived DCs, in the presence or absence of antibodies against LFA-1 (NKI-L15), ALCAM (A2N-L50), and CD6, or recombinant ALCAM-Fc (CD6-Fc). Total mouse IgG (mIgG) and a control Fc-chimeric protein (L-SIGN–Fc) were used as negative controls. Antibodies and Fc-chimeras were added at coculture onset. Proliferation was assessed at day 5 by [3H]-thymidine incorporation for 16 hours. Results are expressed as the percentage of medium control and are the mean ± SD of 3 independent experiments. (C) PBLs (105) were stimulated with 1.7 × 103, 4.5 × 103, or 1.0 × 104 allogeneic mDCs in the presence of anti-CD6. Antibodies were added at coculture onset. Results are expressed as the percentage of proliferation in the presence of control mouse IgG and are the mean ± SD of 3 independent experiments. (D) Mature DC-induced proliferation in the presence of anti–LFA-1 (□), anti-CD6 (▦), or control mIgG (▪) was measured at different time points (days 3, 5, or 7) by [3H]-thymidine incorporation for 16 hours. Antibodies were added at coculture onset. Results are mean ± SD of 3 independent experiments.
ALCAM-CD6 interactions are essential for DC-induced T-cell proliferation. (A) Human PBLs were triple-stained and gated for T cells, NK cells, and B cells. CD6 and ALCAM staining is represented by shaded histograms; isotype-matched controls are represented by open histograms. One representative experiment of 3 is shown. Percentage of positive cells and MFI values are indicated in the top right corner of each subpanel. (B) PBLs (105) were stimulated in vitro with 1.7 × 103 mature monocyte-derived DCs, in the presence or absence of antibodies against LFA-1 (NKI-L15), ALCAM (A2N-L50), and CD6, or recombinant ALCAM-Fc (CD6-Fc). Total mouse IgG (mIgG) and a control Fc-chimeric protein (L-SIGN–Fc) were used as negative controls. Antibodies and Fc-chimeras were added at coculture onset. Proliferation was assessed at day 5 by [3H]-thymidine incorporation for 16 hours. Results are expressed as the percentage of medium control and are the mean ± SD of 3 independent experiments. (C) PBLs (105) were stimulated with 1.7 × 103, 4.5 × 103, or 1.0 × 104 allogeneic mDCs in the presence of anti-CD6. Antibodies were added at coculture onset. Results are expressed as the percentage of proliferation in the presence of control mouse IgG and are the mean ± SD of 3 independent experiments. (D) Mature DC-induced proliferation in the presence of anti–LFA-1 (□), anti-CD6 (▦), or control mIgG (▪) was measured at different time points (days 3, 5, or 7) by [3H]-thymidine incorporation for 16 hours. Antibodies were added at coculture onset. Results are mean ± SD of 3 independent experiments.
Intriguingly, the presence of anti-CD6 antibodies or ALCAM-Fc completely inhibited T-cell proliferation induced by mature DCs (Figure 2B), even to a greater extent than antibodies against LFA-1, known as a major component in costimulation.22,23 Anti-ALCAM antibodies and recombinant CD6-Fc also blocked T-cell proliferation, but to a lesser extent than anti-CD6 and ALCAM-Fc. Proliferation was not affected by control mouse IgG or control Fc–chimeric protein. The blocking effect of anti-CD6 was not overcome by increasing numbers of mDC (Figure 2C). In addition, we measured proliferation at different time points (3, 5, and 7 days) after coculture onset. CD6 and LFA-1 antibodies were added at the beginning of the cocultures (Figure 2D). The presence of CD6 antibodies resulted in a sustained repression of the proliferative response, even at 7 days after addition of the antibodies. In contrast, LFA-1 antibodies inhibited proliferation at early time points but this effect was significantly decreased after 5 days of coculture. These data indicate that ALCAM-CD6–mediated adhesion is important throughout the proliferation process, since the effect of blocking agents can be measured up to 7 days after coculture onset.
CD6 crosslinking enhances anti-CD3–induced T-cell proliferation and activation of NFAT
We next investigated the costimulatory function of CD6 by inducing T-cell proliferation with immobilized anti-CD3, anti-CD6, and anti-CD28, and combinations thereof. Co-crosslinking of CD3 and CD28 expectedly resulted in a strongly enhanced T-cell proliferation compared with CD3 alone (Figure 3A). A similar effect was observed with CD3 and CD6 co-crosslinking. T-cell proliferation was even further enhanced by simultaneous CD3, CD6, and CD28 crosslinking, while CD6 and CD28 alone hardly activated the T cells.
We examined the observed costimulatory effect of CD6 in more detail by nucleofection of Jurkat T cells with an NFAT-luciferase reporter construct, followed by stimulation with immobilized anti-CD3, anti-CD6, and anti-CD28, and combinations thereof. First we determined a suboptimal anti-CD3 concentration (1 μg/mL) at which NFAT activation was still higher than background level (data not shown). In agreement with the observed effects on T-cell proliferation, crosslinking of CD3 together with either CD28 or CD6 resulted in an increased NFAT activation compared with CD3 alone (Figure 3B). Simultaneous crosslinking of CD3 and CD6 also markedly increased activation of NFAT. This CD6-mediated costimulatory effect could be directly correlated with the amount of antibody used. Simultaneous crosslinking of CD3, CD6, and CD28 did not further enhance NFAT activity in the presence of 1 μg/mL of anti-CD3. Apparently, maximal costimulation was reached already with anti-CD28 alone under these conditions. When lower quantities of anti-CD3 (0.2 μg/mL) were used, an additive effect of anti-CD6 and anti-CD28 was observed (data not shown).
CD6 crosslinking enhances T-cell proliferation and activation of NFAT. (A) PBLs (105) were stimulated for 5 days with immobilized anti-CD3 (0.2 μg/mL), anti-CD28 (4 μg/mL), anti-CD6 (5 μg/mL), or combinations thereof. At day 5 proliferation was assessed by [3H]-thymidine incorporation. Mean values ± SEM of 3 independent experiments are shown. (B) Jurkat T cells were transfected with 5 μg of the NFAT-luciferase construct and stimulated for 6 hours with 1 μg/mL immobilized anti-CD3, anti-CD28 (4 μg/mL), anti-CD6 (1 μg/mL or 5 μg/mL), or combinations thereof. Luciferase activities were determined in cell lysates and expressed as percentages of the PMA (10 ng/mL)/ionomycin (1 μM) control. Nontransfected Jurkat cells did not respond to any stimulus (data not shown). Mean values ± SEM of 3 independent experiments are shown. Asterisks indicate significantly higher than anti-CD3 alone (Student t test, *P < .05; **P < .01).
CD6 crosslinking enhances T-cell proliferation and activation of NFAT. (A) PBLs (105) were stimulated for 5 days with immobilized anti-CD3 (0.2 μg/mL), anti-CD28 (4 μg/mL), anti-CD6 (5 μg/mL), or combinations thereof. At day 5 proliferation was assessed by [3H]-thymidine incorporation. Mean values ± SEM of 3 independent experiments are shown. (B) Jurkat T cells were transfected with 5 μg of the NFAT-luciferase construct and stimulated for 6 hours with 1 μg/mL immobilized anti-CD3, anti-CD28 (4 μg/mL), anti-CD6 (1 μg/mL or 5 μg/mL), or combinations thereof. Luciferase activities were determined in cell lysates and expressed as percentages of the PMA (10 ng/mL)/ionomycin (1 μM) control. Nontransfected Jurkat cells did not respond to any stimulus (data not shown). Mean values ± SEM of 3 independent experiments are shown. Asterisks indicate significantly higher than anti-CD3 alone (Student t test, *P < .05; **P < .01).
ALCAM-CD6 interactions are important in the formation of DC–T-cell contacts
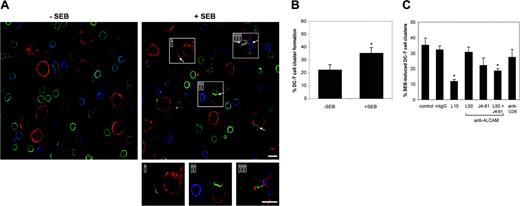
Having assessed the effects of CD6-blocking antibodies and ALCAM-Fc molecules on mature DC-induced T-cell proliferation, we next investigated the role of ALCAM-CD6 interactions at the initial phase of contact between mature DCs and resting T cells in an allogeneic setting. First, we determined the number of DC–T-cell conjugates formed in the presence or absence of the superantigen SEB by confocal microscopy. SEB binds to the α1 domain of the human leukocyte antigen (HLA)–DR1 molecule and is recognized by several isoforms of the human TCR Vβ, thereby implicating 20% of the T lymphocytes.
More DC–T-cell contacts were observed in the presence than in the absence of SEB (Figure 4A-B). Moreover, 3 of 5 SEB-induced DC–T-cell contacts showed enriched levels of CD6 (Figure 4Ai-iii). Approximately 20% of the DCs formed a DC–T-cell conjugate after 15 minutes incubation at 37°C (Figure 4B). Pretreatment of the DCs with SEB increased the number of conjugates to 40% after 15 minutes, indicating that SEB accelerates stable DC–T-cell conjugate formation. After prolonged incubations of 60 minutes, differences between the presence and absence of SEB were no longer observed (data not shown). These findings confirm and extend previous results obtained with conjugates between Jurkat J77 and Raji cells.2 Further experiments were performed with SEB-pretreated DCs and an incubation time of 15 minutes.
To determine if ALCAM-CD6–mediated adhesion is essential for the initiation of antigen-induced DC–T-cell contacts we preincubated the DCs and T cells with blocking antibodies directed against ALCAM (AZN-L50 and J4-81) and CD6 prior to mixing. As a positive control for inhibition we used an LFA-1–blocking antibody. All antibodies remained present during the subsequent DC–T-cell incubations. We also tested the effects of the antibodies on conjugate formation in the absence of SEB and found that none of them significantly reduced the number of antigen-independent DC–T-cell contacts (data not shown). When ALCAM-blocking antibodies AZN-L50 and J4-81 were added separately, no significant effect on SEB-induced DC–T-cell cluster formation was observed (Figure 4C). However, the combination of the 2 antibodies had an additive effect resulting in a significant block (∼50%) of DC–T-cell cluster formation, while anti–LFA-1 antibodies produced a block of about 66%. The presence of the anti-CD6 antibody did not affect the number of conjugates compared with the control situation, which may be explained by the fact that the latter antibody does not bind the ALCAM-binding region (the membrane-proximal SRCR domain) of CD6. This observation contrasts with the strong inhibitory effect of the anti-CD6 antibody on T-cell proliferation and suggests that at least 2 distinct mechanisms are involved in DC–T-cell binding versus T-cell proliferation (“Discussion”). Taken together, these observations point toward an important role for ALCAM-CD6 binding in the formation of DC–T-cell contact.
ALCAM-CD6 interactions are important in the formation of DC–T-cell contacts. (A) Mature monocyte-derived DCs, preincubated with (right panel) or without (left panel) 1 μg/mL SEB, were incubated with PBLs of an unrelated donor in a 1:10 ratio for 15 minutes at 37°C, allowing for DC–T-cell cluster formation. After mounting on poly(l)lysine–coated slides DCs were stained for ALCAM (red), and PBLs were stained for CD6 (green) and LFA-1 (blue). Arrows indicate DC–T-cell conjugates. Magnifications of 3 mature DC–T-cell contacts are shown (subpanels i-iii). Scale bar represents 10 μm. (B) The number of DC–T-cell contacts in the presence or absence of SEB was determined by counting 20 randomly selected microscopic fields. At least 100 DCs per condition were analyzed. The percentage of DC–T-cell conjugates per total number of DCs is calculated. Mean ± SEM of 6 independent experiments is shown. (C) Prior to mixing, DCs (SEB-preincubated) and PBLs were incubated with 20 μg/mL antibody against LFA-1 (L15), ALCAM (AZN-L50 and/or J4-81), anti-CD6, or control mouse IgG for 30 minutes on ice. Antibodies remained present during conjugate formation. The percentage of conjugates was calculated as described for panel B. Mean ± SEM of 6 independent experiments is shown. *Statistically significant differences (Student t test, P < .05).
ALCAM-CD6 interactions are important in the formation of DC–T-cell contacts. (A) Mature monocyte-derived DCs, preincubated with (right panel) or without (left panel) 1 μg/mL SEB, were incubated with PBLs of an unrelated donor in a 1:10 ratio for 15 minutes at 37°C, allowing for DC–T-cell cluster formation. After mounting on poly(l)lysine–coated slides DCs were stained for ALCAM (red), and PBLs were stained for CD6 (green) and LFA-1 (blue). Arrows indicate DC–T-cell conjugates. Magnifications of 3 mature DC–T-cell contacts are shown (subpanels i-iii). Scale bar represents 10 μm. (B) The number of DC–T-cell contacts in the presence or absence of SEB was determined by counting 20 randomly selected microscopic fields. At least 100 DCs per condition were analyzed. The percentage of DC–T-cell conjugates per total number of DCs is calculated. Mean ± SEM of 6 independent experiments is shown. (C) Prior to mixing, DCs (SEB-preincubated) and PBLs were incubated with 20 μg/mL antibody against LFA-1 (L15), ALCAM (AZN-L50 and/or J4-81), anti-CD6, or control mouse IgG for 30 minutes on ice. Antibodies remained present during conjugate formation. The percentage of conjugates was calculated as described for panel B. Mean ± SEM of 6 independent experiments is shown. *Statistically significant differences (Student t test, P < .05).
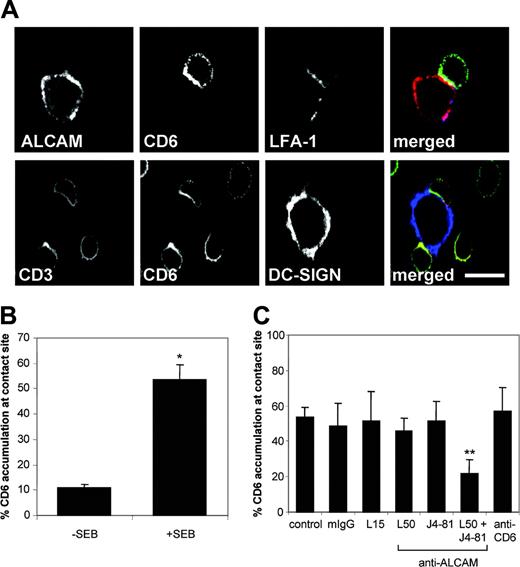
Both ALCAM and CD6 are recruited at the contact site between T cells and DCs
A more detailed confocal analysis of the interface between mature DCs and resting T cells is presented in Figure 5. ALCAM and CD6 accumulated and colocalized at the contact site between mature DCs and naive T cells in the presence of SEB (Figure 5A). As expected, LFA-1 was also detected at the contact area. CD6 colocalized at least partially with CD3 at the T-cell surface. In agreement with recent data24 DC-SIGN, an important counterreceptor for ICAM-3,25 did not concentrate at the DC–T-cell interface (Figure 5A). The accumulation of CD6 in the immunological synapse was markedly enhanced in the presence of SEB (Figure 5B) and generally coincided with CD3 accumulation (data not shown). We subsequently analyzed the effect of blocking antibodies on the accumulation of CD6 at the DC–T-cell interface (Figure 5C). Again, DCs and T cells were preincubated with blocking antibodies, which remained present during cluster formation. In the control situation, CD6 accumulation occurred in about 55% of the DC–T-cell conjugates. Of the conjugates that were formed in the presence of ALCAM-blocking antibodies, only 20% still showed enriched CD6 levels at the contact site. The presence of anti–LFA-1 antibodies significantly reduced the number of T-cell–APC conjugates (Figure 4C). However, in the remaining contacts CD6 accumulation in the synapse was present to the same extent as in the control situation (Figure 5C). These results suggest that CD6 is recruited toward the synapse independently of LFA-1.
CD6 and ALCAM accumulate at the contact site between DCs and T cells in the presence of SEB. (A) Mature DCs were preincubated with 1 μg/mL SEB and subsequently mixed with PBLs of an unrelated donor in a 1:10 ratio for 15 minutes at 37°C. Cell conjugates were mounted on poly(l)lysine–coated slides and stained for ALCAM (red), CD6 (green), and LFA-1 (blue), or DC-SIGN (blue), CD6 (green), and CD3 (red). Scale bar represents 10 μm. (B) The percentage of CD6 accumulation at the contact site was scored relative to the total number of DC–T-cell contacts counted. Mean ± SEM of 6 independent experiments is shown. (C) Prior to mixing, DCs (SEB-preincubated) and PBLs were incubated with 20 μg/mL antibody against LFA-1 (L15), ALCAM (L50 and/or J4-81), anti-CD6, or control mouse IgG for 30 minutes on ice. Antibodies remained present during conjugate formation. The percentage of CD6 accumulation was calculated as described for panel B. Mean ± SEM of 6 independent experiments is shown. Asterisks indicate statistically significant differences (Student t test, *P < .001; **P < .05).
CD6 and ALCAM accumulate at the contact site between DCs and T cells in the presence of SEB. (A) Mature DCs were preincubated with 1 μg/mL SEB and subsequently mixed with PBLs of an unrelated donor in a 1:10 ratio for 15 minutes at 37°C. Cell conjugates were mounted on poly(l)lysine–coated slides and stained for ALCAM (red), CD6 (green), and LFA-1 (blue), or DC-SIGN (blue), CD6 (green), and CD3 (red). Scale bar represents 10 μm. (B) The percentage of CD6 accumulation at the contact site was scored relative to the total number of DC–T-cell contacts counted. Mean ± SEM of 6 independent experiments is shown. (C) Prior to mixing, DCs (SEB-preincubated) and PBLs were incubated with 20 μg/mL antibody against LFA-1 (L15), ALCAM (L50 and/or J4-81), anti-CD6, or control mouse IgG for 30 minutes on ice. Antibodies remained present during conjugate formation. The percentage of CD6 accumulation was calculated as described for panel B. Mean ± SEM of 6 independent experiments is shown. Asterisks indicate statistically significant differences (Student t test, *P < .001; **P < .05).
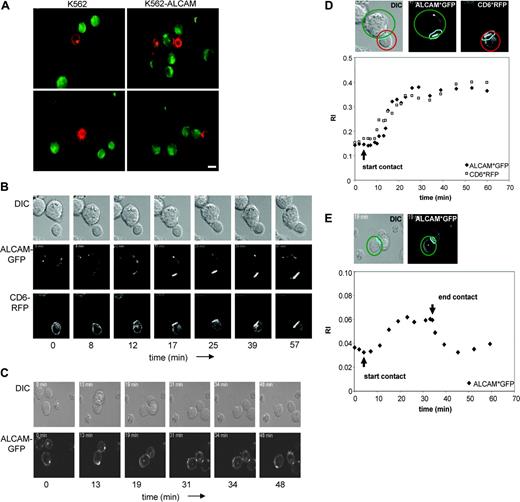
Dynamic redistribution of ALCAM and CD6 at the T-cell–APC interface
The dynamic behavior of ALCAM and CD6 at the T-cell–APC contact site was studied by time-lapse confocal microscopy using Jurkat-CD6*RFP T cells and K562-ALCAM*GFP. Since K562 cells express moderate levels of HLA-DR/DP and CD80 (data not shown), they were used as a model APC in this experimental, antigen-independent setup. Interactions between Jurkat-CD6*RFP and K562-ALCAM*GFP were indeed ALCAM mediated, since no conjugates were observed between Jurkat-CD6*RFP and parental K562 cells (Figure 6A). Both ALCAM and CD6 redistributed to the contact area between Jurkat-CD6*RFP and K562-ALCAM*GFP (Figure 6B). All initial Jurkat-CD6*RFP/K562-ALCAM*GFP contacts that gave rise to a stable conjugate were preceded by accumulation of ALCAM and CD6 at the cell-cell interface (Videos S1, S2). We quantified the enrichment of ALCAM and CD6 at the contact site by calculating the recruitment index (RI), as indicated in Figure 6D, top panel. Both molecules started to accumulate 6 minutes after initial contact. Their maximal RI value (∼2.5-fold increase compared with the starting situation) was reached after 20 minutes and lasted for at least 60 minutes (Figure 6D). These findings show that ALCAM-CD6 binding is robust and implicate that ALCAM-CD6 mediated cell-cell interactions are also stable in vivo, which may relate to the observed long-lasting effects on T-cell proliferation.
As known from previous reports, ALCAM not only interacts with CD6 but it also mediates homotypic ALCAM-ALCAM adhesion.7,9,10 Accordingly, we observed homotypic ALCAM-ALCAM interactions between K562-ALCAM*GFP cells (Figure 6C). In contrast to ALCAM-CD6 binding, ALCAM-ALCAM interactions were transient and accumulation of ALCAM rapidly decreased (within 1-2 minutes) when cells disengaged (Figure 6E, Video S3). From the initial cell-cell contact, ALCAM-ALCAM interactions showed recruitment kinetics similar to ALCAM-CD6. Recruitment started within 8 minutes after initial contact and was maximal within 20 minutes. However, the relative enrichment of ALCAM in these contacts, again expressed as RI, was significantly lower compared with ALCAM-CD6 interactions. These findings implicate that cell-cell interactions mediated via ALCAM-ALCAM are less strong in vivo compared with ALCAM-CD6.
Discussion
The work presented in this paper builds on previous observations by Gimferrer and colleagues, who first showed that CD6 colocalizes with CD3 at the center of the immunologic synapse and is involved in interactions needed for synapse maturation.20 Here we confirm and extend their findings by demonstrating that CD6-ALCAM interactions are involved in both stabilization of DC–T-cell contact and the second, proliferative phase of the T-cell response. T-cell proliferation is sustained for days by CD6-ALCAM binding, in which CD6 serves as an important costimulatory molecule. Both CD6 and ALCAM are actively recruited at the DC–T-cell interface and localize all along the contact site. In contrast to transient, homotypic ALCAM-ALCAM interactions, CD6-ALCAM binding is robust and long-lasting, supporting the long-term effects observed on T-cell proliferation.
Although comparable levels of ALCAM are present on immature and mature DCs, we observed a higher binding of mature DCs to CD6-Fc–coated beads. This may be due to differences in plasma membrane distribution of ALCAM molecules induced by maturation. During DC development, rearrangement of receptor organization at the cell surface can occur, as has been reported for the C-type lectin DC-SIGN.26 Dynamic redistribution of LFA-1 into clusters at the cell membrane increases its ligand binding potential.27,28 This phenomenon, known as avidity regulation, has also been implicated in ALCAM-mediated adhesion.9,10 The molecular organization of ALCAM at the DC surface at different developmental stages is currently under investigation.
Upon antigen-induced conjugate formation, CD6 and ALCAM are redistributed to the T-cell–DC interface. CD6 partially colocalizes with CD3, in agreement with the previous finding that CD6 and CD3 colocalize in the central SMAC of the immunological synapse between Jurkat J77 and Raji B cells.20 However, in most of the analyzed conjugates we found CD6 accumulation all along the contact region, suggesting that the presence of CD6 is not restricted to the cSMAC and that the peripheral SMAC also contains CD6. The localization of CD6 in both SMACs may relate to a dual function for CD6. It is tempting to speculate that CD6 in the pSMAC contributes to contact stabilization, similar to LFA-1, which is known to localize to the pSMAC upon synapse maturation.4 CD6 in the cSMAC might be involved in costimulation, which is supported by its colocalization with CD3 and its association with the costimulatory molecule CD5.18
Dynamic redistribution of ALCAM and CD6 during the formation of T-cell–APC conjugates. (A) Jurkat-CD6*RFP cells were mixed with CFSE-labeled K562 or K562-ALCAM cells in a 1:1 ratio and incubated for 20 minutes at 37°C. Cells were mounted on poly(l)lysine coated slides and analyzed by fluorescence microscopy. Scale bar represents 10 μm. (B-C) Jurkat-CD6*RFP cells were allowed to adhere onto fibronectin-coated slides and K562-ALCAM*GFP cells were added in a 1:2 ratio. Each time point shows the DIC images merged with the fluorescence images of the Jurkat and K562 cells taken from the video at the indicated time points. Time frames were selected based on clear redistribution events. Representative examples of ALCAM and CD6 relocalization at the Jurkat/K562-ALCAM interface (B) and the K562-ALCAM/K562-ALCAM interface (C) are shown. (D-E) Fluorescence intensity of ALCAM*GFP (♦) and CD6*RFP (▪) in a fixed region of the synapse between Jurkat and K562-ALCAM (D) or 2 K562-ALCAM cells (E). The fluorescence intensity in the synapse region (light blue) was divided by the average intensity measured in the whole cell (ALCAM-expressing K562 cells represented in green and CD6-expressing Jurkat cells in red), as indicated in the top panels. These values, representing the recruitment index (RI), were calculated for each imaging time point and plotted on the diagrams. RI values of 1 representative conjugate (out of 3 independent experiments) are shown.
Dynamic redistribution of ALCAM and CD6 during the formation of T-cell–APC conjugates. (A) Jurkat-CD6*RFP cells were mixed with CFSE-labeled K562 or K562-ALCAM cells in a 1:1 ratio and incubated for 20 minutes at 37°C. Cells were mounted on poly(l)lysine coated slides and analyzed by fluorescence microscopy. Scale bar represents 10 μm. (B-C) Jurkat-CD6*RFP cells were allowed to adhere onto fibronectin-coated slides and K562-ALCAM*GFP cells were added in a 1:2 ratio. Each time point shows the DIC images merged with the fluorescence images of the Jurkat and K562 cells taken from the video at the indicated time points. Time frames were selected based on clear redistribution events. Representative examples of ALCAM and CD6 relocalization at the Jurkat/K562-ALCAM interface (B) and the K562-ALCAM/K562-ALCAM interface (C) are shown. (D-E) Fluorescence intensity of ALCAM*GFP (♦) and CD6*RFP (▪) in a fixed region of the synapse between Jurkat and K562-ALCAM (D) or 2 K562-ALCAM cells (E). The fluorescence intensity in the synapse region (light blue) was divided by the average intensity measured in the whole cell (ALCAM-expressing K562 cells represented in green and CD6-expressing Jurkat cells in red), as indicated in the top panels. These values, representing the recruitment index (RI), were calculated for each imaging time point and plotted on the diagrams. RI values of 1 representative conjugate (out of 3 independent experiments) are shown.
Recruitment of CD6 and ALCAM at the T-cell–APC interface started 6 minutes after contact initiation, similar to LFA-1 which is recruited within 4 minutes in the pSMAC.24 However, CD6 accumulation in the synapse occurs independently of LFA-1 redistribution, since its relocalization was not affected by anti–LFA-1 antibodies. CD6 recruitment occurs significantly later than ICAM-3 recruitment, which relocalizes in the synapse within 30 seconds.2 ICAM-3 is already recruited before antigen-specific recognition occurs and induces MHC-II clustering into the immunological synapse.24 In contrast to ICAM-3, CD6 recruitment appears to be at least partially antigen dependent since CD6 enrichment at the T-cell–DC interface is markedly enhanced in the presence of antigen. Thus unlike ICAM-3, CD6 and ALCAM do not appear to be involved in the initial APC surface-scanning phase, but rather in stabilizing T-cell–APC contacts after TCR-MHC binding has occurred.
Previous reports have pointed toward a role for CD6-mediated interactions in facilitating T-cell–APC contact.17,20 Here we show that inhibition of CD6-ALCAM binding results in a markedly decreased number of T-cell–DC conjugates. Blocking of LFA-1–ICAM-1–mediated adhesion also strongly interferes with contact formation (Figure 4), which indicates that both receptor-ligand pairs are important in mediating contact between resting T cells and APCs. Our video images of Jurkat-CD6*RFP and K562-ALCAM*GFP cells demonstrate that ALCAM-CD6 mediated interactions are stable and long lasting, supporting a role in long-term adhesion in vivo. In contrast, homotypic ALCAM-ALCAM interactions are more transient and show less recruitment of ALCAM compared with ALCAM-CD6 binding. ALCAM-ALCAM–mediated contacts are unlikely to contribute to DC-induced activation of resting T cells, since only activated T lymphocytes express significant levels of ALCAM.
Allogeneic DC–induced T-cell proliferation is effectively inhibited by ALCAM- and CD6-blocking antibodies as well as recombinant ALCAM-Fc and CD6-Fc proteins, pointing toward an important role for ALCAM-CD6–mediated adhesion in T-cell activation. Consistent with our findings, recombinant monomeric CD6 and ALCAM chimeras were shown to inhibit cell proliferation induced by the tetanus toxoid Ag.17 We observed interesting differences between the effects of antibodies against CD6 and LFA-1, an adhesion molecule known to play a role in stabilization of the immunological synapse and T-cell costimulation.22,29 While CD6 blockade resulted in a complete inhibition of allogeneic DC–induced T-cell proliferation, LFA-1 antibodies had only a partial effect. Furthermore, the anti-CD6 induced block of proliferation was sustained for at least 7 days, whereas the inhibitory effect of LFA-1 antibodies significantly decreased 3 days after initiation of the T-cell–DC coculture. These findings indicate that either the costimulatory signal provided by LFA-1 is short-lived or LFA-1 is merely involved in mediating contact. It has been reported that LFA-1 exerts its costimulatory function by facilitating and prolonging contact between an APC and T or B cell rather than by reducing the minimal number of TCRs that have to be triggered, as was reported for the prominent costimulatory receptor CD28.30-32 We show here that CD6 displays functional similarity with CD28, since CD6 crosslinking enhances CD3-induced proliferation and activation of NFAT comparable to CD28 crosslinking. These results validate and extend previous findings that simultaneous CD6 and CD3 crosslinking increases intracellular calcium levels and T-cell proliferation.33-35 Although CD28 is generally appreciated as the primary costimulatory receptor used by APCs to augment T-cell proliferation,36,37 we now show that CD6 blockade completely impaired DC-induced T-cell proliferation up to 7 days after activation. Thus, the function of CD6 may comprise the long-term maintenance and/or elevation of existing costimulatory signals, whereas CD28 may boost T-cell costimulation at the early activation phase of the immune response.
Interestingly, the cytoplasmic domain of CD6 resembles that of CD28. Both contain multiple tyrosine residues that are not part of an immunoreceptor tyrosine-based activation motif (ITAM) and proline-rich motifs that are able to recruit kinases of different families. Lck and Itk have been shown to bind to proline-rich motifs of both CD6 and CD28.19,32 However, Lck and Itk association with LFA-1 has not been reported. Itk activity is regulated by Lck38 and is crucial for the production of second messengers that are required for a strong and sustained T-cell–activating signal. Furthermore, it has been proposed that CD6 regulates tyrosine phosphorylation of CD5, another molecule involved in the regulation of signal transduction in T lymphocytes, by recruiting Lck, Itk, Fyn, and ZAP-70 kinases.19 Simultaneous association of different kinases that increase each other's activities may explain the strong effects of CD6 crosslinking we observed on T-cell signaling and proliferation.
Association and cooperation of CD6 and CD5 may relate to our finding that the presence of CD6-blocking antibody that does not bind the ALCAM-binding region of CD6, still results in a dramatic block of T-cell proliferation. We speculate that 2 different mechanisms involving CD6 may be operative during T-cell activation and costimulation. First, CD6-ALCAM binding stabilizes DC–T-cell contact and triggers CD6 activation. ALCAM antibodies can effectively inhibit this step in vitro. Secondly, CD6 physically associates with CD5 at the plasma membrane and regulates CD5 tyrosine phosphorylation.19 The presence of the anti-CD6 antibody may interfere with CD6-CD5 association and abrogate CD6-induced phosphorylation of CD5. It will therefore be interesting to study the effects of CD6 blockade on CD5-induced signaling pathways.
Taken together, the present study demonstrates that CD6-ALCAM interactions play a pivotal role in stabilizing the T-cell–APC contacts. CD6 has a dual function in that it both facilitates stable adhesion and acts as a costimulatory molecule. Once the antigen-specific TCR-MHC contact has been established, CD6 is rapidly recruited to the T-cell–APC interface, where it stabilizes the immunological synapse by interacting with ALCAM. Subsequent CD6-modulated signaling events result in a strong and sustained costimulatory signal that is essential for T-cell proliferation. Future research will be aimed at unraveling the molecular mechanisms underlying CD6-mediated signal transduction in order to fully understand its role as a costimulatory molecule.
Prepublished online as Blood First Edition Paper, December 13, 2005; DOI 10.1182/blood-2005-09-3881.
Supported by grants from the Radboud University Nijmegen Medical Centre (A.W.Z.) and the Netherlands Organisation for Scientific Research (C.G.F.).
A.W.Z. performed mixed lymphocyte reactions, NFAT, beads binding and conjugate formation assays, FACS analysis, and cell transfections; B.J. performed live video imaging; R.T. generated the ALCAM antibody AZN-L50; J.R.P. cloned and sequenced CD6 full-length cDNA; A.W.Z., F.N.v.L., and C.G.F. designed the studies; and A.W.Z. wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr T. Jovin (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for providing the monomeric RFP cDNA construct, Carla Schwarte for help with beads binding experiments, and Dr A. van Spriel and Dr F. de Lange for critically reading the manuscript. The Microscopic Imaging Centre (MIC) of the NCMLS is kindly acknowledged for its technical support.

![Figure 2. ALCAM-CD6 interactions are essential for DC-induced T-cell proliferation. (A) Human PBLs were triple-stained and gated for T cells, NK cells, and B cells. CD6 and ALCAM staining is represented by shaded histograms; isotype-matched controls are represented by open histograms. One representative experiment of 3 is shown. Percentage of positive cells and MFI values are indicated in the top right corner of each subpanel. (B) PBLs (105) were stimulated in vitro with 1.7 × 103 mature monocyte-derived DCs, in the presence or absence of antibodies against LFA-1 (NKI-L15), ALCAM (A2N-L50), and CD6, or recombinant ALCAM-Fc (CD6-Fc). Total mouse IgG (mIgG) and a control Fc-chimeric protein (L-SIGN–Fc) were used as negative controls. Antibodies and Fc-chimeras were added at coculture onset. Proliferation was assessed at day 5 by [3H]-thymidine incorporation for 16 hours. Results are expressed as the percentage of medium control and are the mean ± SD of 3 independent experiments. (C) PBLs (105) were stimulated with 1.7 × 103, 4.5 × 103, or 1.0 × 104 allogeneic mDCs in the presence of anti-CD6. Antibodies were added at coculture onset. Results are expressed as the percentage of proliferation in the presence of control mouse IgG and are the mean ± SD of 3 independent experiments. (D) Mature DC-induced proliferation in the presence of anti–LFA-1 (□), anti-CD6 (▦), or control mIgG (▪) was measured at different time points (days 3, 5, or 7) by [3H]-thymidine incorporation for 16 hours. Antibodies were added at coculture onset. Results are mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-09-3881/4/m_zh80080693970002.jpeg?Expires=1769937570&Signature=DubWVsMjqRZ42zpt665qTxxwA2B01zp8vaA5gj-IpWGMYtbAgaF7OeA~X2dcnEEXJOOeRHVRSKIWLzmlTeaOTcBikmZsttWGGYnhR5GEtQlsCfRRON43Kwxkf-e7dgFQoqkDlpNtPWfTuiJDek2GX3hHyNlAFcvRdPZ7izRLai2z1KV7fWK-mTVAtsVNTPPlyYNv8ExJkoxGUVtmc0QdJSPyj34IrjQs2a1lXn~axxugLJ7QfOb~eASvEyuPm5n5MS1IcMdbtfL~8~QD-6qNfNKwMC8JldPi6Jl5AV3~BCEsks59-La4X1p1OJ~j9W2~3fjoFUNA67BGaAhMucfnqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. CD6 crosslinking enhances T-cell proliferation and activation of NFAT. (A) PBLs (105) were stimulated for 5 days with immobilized anti-CD3 (0.2 μg/mL), anti-CD28 (4 μg/mL), anti-CD6 (5 μg/mL), or combinations thereof. At day 5 proliferation was assessed by [3H]-thymidine incorporation. Mean values ± SEM of 3 independent experiments are shown. (B) Jurkat T cells were transfected with 5 μg of the NFAT-luciferase construct and stimulated for 6 hours with 1 μg/mL immobilized anti-CD3, anti-CD28 (4 μg/mL), anti-CD6 (1 μg/mL or 5 μg/mL), or combinations thereof. Luciferase activities were determined in cell lysates and expressed as percentages of the PMA (10 ng/mL)/ionomycin (1 μM) control. Nontransfected Jurkat cells did not respond to any stimulus (data not shown). Mean values ± SEM of 3 independent experiments are shown. Asterisks indicate significantly higher than anti-CD3 alone (Student t test, *P < .05; **P < .01).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-09-3881/4/m_zh80080693970003.jpeg?Expires=1769937570&Signature=CWIcFkKGx~uijHnOG5rIi7csgE9sVaZXSzxRTpmdCGM0N7SLSnEWg5ZdL9DvygmBk1NbOOA7~Mv0q~Ntw3gBAvRgxUKEoMZU8Xvjx3-FXwmDv6Agyo8NA7wAMMUsRAqwffBlS3l7l~oXYgUkHBXJjyJjm1wtGeYUg35ZOuv6JeDiy23yBIUIKEG0y0vnhZ-42KflMO-VSA~ctWrYC8dKD4Ak1iDjwM92X3MUR2AHePfbipft4zYWIev7dSaoprZZCwQxEcR996MnNGpp-GKnyO3u~DNyPnnjzox8k99Rt8mQTiNPuA59MGek3RwBXKJqumRW3vVbMV6s2Jh3FS3Slw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal