Abstract
Innate CD1d-restricted natural killer T (NKT) cells are infected and lost in HIV-1–infected patients, and this could contribute to HIV-1 pathogenesis because NKT cells play an important role in directing both adaptive and innate immunity. Administration of interleukin-2 (IL-2) to HIV-1–infected patients leads to substantial and sustained CD4+ T-cell expansion, involving both naive and memory cells. We investigated whether IL-2 treatment could restore the NKT cell compartment in patients with primary HIV-1 infection. We show that IL-2 combined with effective antiretroviral therapy (ART) resulted in significant expansion of CD1d-restricted NKT cells. Expansion occurred in both the CD4– and CD4+ subsets of NKT cells, and expanded cells expressed the CD161 maturation marker while expression of the HIV coreceptor CCR5 was reduced. These data indicate that IL-2 treatment in combination with effective ART is beneficial for the restoration of innate NKT cell immunity in patients with primary HIV-1 infection.
Introduction
Human Vα24 natural killer T (NKT) cells are a CD1d-restricted T-lymphocyte lineage with immunoregulatory functions operating on the border between the innate and the adaptive immune system. They are characterized by coexpression of an invariant and conserved αβ T-cell receptor (TCR) and the NK cell marker CD161, and recognize endogenous and exogenous glycolipid antigens presented by CD1d.1-3 Recently, we and others have reported that NKT cells are targets of HIV-1 infection.4-6 NKT cells are reduced in peripheral blood mononuclear cell (PBMC) cultures infected with HIV-1 in vitro, and depleted in peripheral blood of HIV-1–infected subjects with uncontrolled viremia. Because these cells are mainly targeted by CCR5-using HIV-1 strains,4,5 NKT cells might be early targets of HIV-1 and this could therefore play an important role in establishing HIV-1 infection. Loss of NKT cells in HIV-1–infected subjects may have several pathologic consequences, including impaired tumor immunity and impaired immune responses against opportunistic infections.
Several clinical trials have shown that combination of antiretroviral treatment (ART) with interleukin-2 (IL-2) therapy is beneficial for HIV-1–infected patients. In both chronic and primary infection, administration of IL-2 leads to substantial CD4+ T-cell expansions while maintaining undetectable viral loads.7-11 Moreover, CD4+ T-cell expansions can be lastingly maintained by low frequency IL-2 administration.12 Expanded cells comprise naive and memory CD4+ T cells, as well as foxP3 positive, but poorly suppressive, CD4+CD25+ T cells.13 In the present study we investigated the effect of IL-2 therapy in combination with effective ART on the NKT cell compartment in primary HIV-1 infection.
Study design
Study subjects
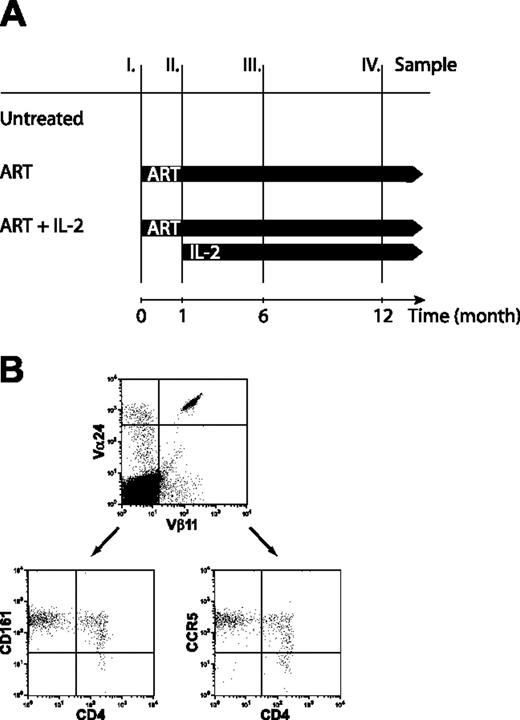
Subjects were from the University of California San Francisco (UCSF) Options Project study of acute and early HIV-1 infection, and were screened as described.14 All patients were ART-naive before entering the study, and were offered a standard ART regimen. Those who chose to begin ART were offered the opportunity to participate in a randomized, controlled trial of adding IL-2 to ART. To qualify for the IL-2 trial, subjects had to reach HIV-1 RNA levels below 500 copies/mL. Patients who met this threshold were randomized to receive IL-2 within 1 month or to be part of a control group that could receive IL-2 after 48 weeks of ART alone. IL-2 was given as a subcutaneous injection (7.5 million units, twice daily) for 5 days at 8-week intervals, with dose adjustments made for toxicity. Whole-blood samples were collected at month 0 (prior to ART treatment), before initiation of IL-2 therapy (month 1), and after completion of 2 (month 6) and 5 (month 12) cycles of IL-2 treatment (Figure 1A). Three groups of patients were investigated: 10 patients in the IL-2 trial who were randomized to receive ART and IL-2 treatment; 11 subjects in the IL-2 trial who were randomized to ART alone for the first 12 months; and 11 subjects in the Options Project who elected not to take any ART. Patients were similar in age and sex; the untreated patients were less frequently enrolled before seroconversion, but this difference was not statistically significant (Fisher exact test; Table 1). The study was approved by the UCSF Committee for Human Research, and patients gave signed informed consent.
Study outline and NKT cell phenotyping. (A) Thirty-two subjects with primary HIV-1 infection were enrolled in 3 different treatment groups. Twenty-one subjects started ART; 10 went on additional IL-2 treatment once their HIV-1 RNA level was below 500 copies/mL. Eleven subjects remained untreated. Whole-blood samples were collected at month 0 (prior to ART treatment), month 1 (before initiation of IL-2), and at months 6 and 12 (after completion of 2 and 5 cycles of IL-2 treatment, respectively). Blood samples were drawn in all patient groups at the stated time points. (B) NKT cells identified by coexpression of Vα24 and Vβ11 express CD161 and CCR5 on both the CD4+ and CD4– subset.
Study outline and NKT cell phenotyping. (A) Thirty-two subjects with primary HIV-1 infection were enrolled in 3 different treatment groups. Twenty-one subjects started ART; 10 went on additional IL-2 treatment once their HIV-1 RNA level was below 500 copies/mL. Eleven subjects remained untreated. Whole-blood samples were collected at month 0 (prior to ART treatment), month 1 (before initiation of IL-2), and at months 6 and 12 (after completion of 2 and 5 cycles of IL-2 treatment, respectively). Blood samples were drawn in all patient groups at the stated time points. (B) NKT cells identified by coexpression of Vα24 and Vβ11 express CD161 and CCR5 on both the CD4+ and CD4– subset.
Subject characteristics
Characteristic . | IL-2+ART . | ART alone . | Untreated . |
|---|---|---|---|
| No. subjects | 10 | 11 | 11 |
| Initial HIV-1 RNA, median (range), log10 copies/mL | 4.9 (4.3-5.7) | 5.8 (5.3-6.2) | 4.9 (4.2-5.5) |
| Initial CD4+ T cells, median (range), cells/μL | 483 (324-638) | 452 (255-519) | 499 (376-593) |
| Male, % | 90 | 100 | 87 |
| Age, median (range), y | 34 (26-56) | 36 (24-47) | 37 (34-50) |
| Median estimated time from infection to treatment, d | 91 | 86 | NA |
| Preseroconversion at enrollment, % | 20 | 45 | 9 |
| PI-containing ART regimen, % | 40 | 64 | NA |
Characteristic . | IL-2+ART . | ART alone . | Untreated . |
|---|---|---|---|
| No. subjects | 10 | 11 | 11 |
| Initial HIV-1 RNA, median (range), log10 copies/mL | 4.9 (4.3-5.7) | 5.8 (5.3-6.2) | 4.9 (4.2-5.5) |
| Initial CD4+ T cells, median (range), cells/μL | 483 (324-638) | 452 (255-519) | 499 (376-593) |
| Male, % | 90 | 100 | 87 |
| Age, median (range), y | 34 (26-56) | 36 (24-47) | 37 (34-50) |
| Median estimated time from infection to treatment, d | 91 | 86 | NA |
| Preseroconversion at enrollment, % | 20 | 45 | 9 |
| PI-containing ART regimen, % | 40 | 64 | NA |
Comparisons across groups and pairwise comparisons between treated and untreated groups all P > .1.
NA indicates not applicable.
Samples
PBMCs were isolated from whole blood, cryopreserved, and stored at the UCSF AIDS Specimen Bank. Thawed samples were washed and cultured overnight before analysis by flow cytometry. Plasma HIV-1 RNA levels were measured using the Bayer branched-chain DNA test version 3.0 (Bayer, Emeryville, CA). T-cell counts were performed by Unilab (San Jose, CA).
Flow cytometry
Vα24 NKT cell frequency and expression of surface markers were assessed in PBMCs by 4-color flow cytometry using the following monoclonal antibodies: Vα24-phycoerythrin and Vβ11-fluorescein isothiocyanate (Immunotech, Marseilles, France); CD4-peridinin chlorophyll protein, CD161-allophycocyanin (APC), and CCR5-APC (BD Pharmingen, San Diego, CA). Samples were analyzed on a FACSCalibur (BD Pharmingen) instrument using CellQuest software. Changes in cell numbers and other measures over time were assessed within treatment groups using the Wilcoxon signed rank test using Sigma Stat software (SPSS, Chicago, IL).
Results and discussion
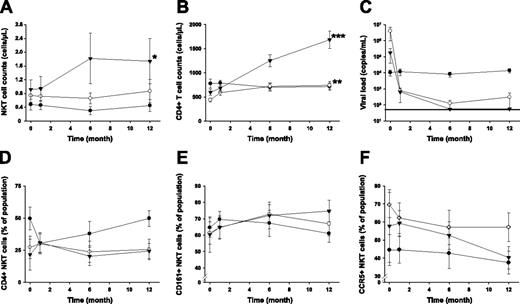
Of a total of 32 patients with primary HIV-1 infection enrolled in this study, 10 patients received intermittent subcutaneous IL-2 on the background of effective ART, 11 patients received only ART, and 11 patients remained untreated. Patients in the IL-2 group received up to 5 IL-2 treatment cycles during the study period. NKT cells in PBMCs were identified by coexpression of the Vα24 and Vβ11 TCR α- and β-chain segments and further phenotyped for expression of CD4, CD161, and CCR5 (Figure 1B). These measurements revealed that the NKT cell compartment responded to IL-2 administration, and we observed a significant increase in mean NKT cell numbers (90% increase; P = .04) from baseline (month 0) to the end of the study period (month 12) (Figure 2A). Subjects receiving IL-2 in combination with ART doubled their NKT cell counts after administration of 2 cycles of IL-2. Additional IL-2 injections resulted in further increasing or stabilizing NKT cell counts. In one patient in whom IL-2 treatment was stopped after 2 cycles, the initially increased NKT counts dropped back to pre–IL-2 treatment levels, suggesting that a sustained effect may require more than 2 cycles of IL-2 (data not shown). Patients treated with ART alone showed a weak, statistically insignificant trend toward an increase in NKT cell counts over the duration of the study (17.8% increase, P = .59), whereas NKT counts in untreated patients decreased slightly by 6.1% (P = .88; Figure 2A). Importantly, although both ART-only and ART+IL-2 treatment resulted in a significant increase in general CD4+ T-cell counts (69.3%, P = .003 and 182.5%, P < .001, respectively; Figure 2B), only IL-2–treated subjects showed a significant increase in the NKT cell compartment. Thus, the expansion of NKT cells in response to IL-2 treatment cannot be explained by the general expansion of T cells but rather by effects specifically stimulating the expansion or de novo production of NKT cells. IL-2 may not exert a direct effect on NKT cells since expression of the IL-2 receptor α-chain is not readily detectable on these cells.15 One may speculate that the effects of IL-2 on other immune cells, such as dendritic cells, could indirectly stimulate the expansion of NKT cells. Notably, the addition of IL-2 to the ART regimen had no negative effect on the suppression of viral replication (Figure 2C).
IL-2 treatment restores the NKT cell compartment in primary HIV-1–infected subjects. Longitudinal measurement of (A) mean NKT cell counts, (B) mean CD4+ T-cell counts, (C) mean viral loads, (D-F) the proportion of CD4, CD161, and CCR5 expressing NKT cells in ART+IL-2–treated (▾), ART-treated (○), and untreated subjects (•). Standard errors are indicated. *P < .05; **P < .005; and ***P < .001, as determined by Wilcoxon signed rank test, indicating statistically significant differences comparing data at baseline and the end of the study period within the respective groups. Horizontal line in panel C indicates limit of detection.
IL-2 treatment restores the NKT cell compartment in primary HIV-1–infected subjects. Longitudinal measurement of (A) mean NKT cell counts, (B) mean CD4+ T-cell counts, (C) mean viral loads, (D-F) the proportion of CD4, CD161, and CCR5 expressing NKT cells in ART+IL-2–treated (▾), ART-treated (○), and untreated subjects (•). Standard errors are indicated. *P < .05; **P < .005; and ***P < .001, as determined by Wilcoxon signed rank test, indicating statistically significant differences comparing data at baseline and the end of the study period within the respective groups. Horizontal line in panel C indicates limit of detection.
Expansion of the NKT cell compartment in IL-2–treated subjects occurred in both CD4+ and CD4– subsets of cells, in that no significant changes in the CD4+/CD4– ratio within the NKT cell compartment could be observed (Figure 2D). NKT cells in the IL-2–treated group showed a trend toward an increasing proportion of CD161+ cells, indicating maturation of NKT cells (P = .05; Figure 2E).16-18 Furthermore, in IL-2–treated patients the proportion of NKT cells expressing the HIV-1 coreceptor CCR5 decreased over time (Figure 2F). This decrease occurred in both CD4+ and CD4– subsets of NKT cells but was mainly driven by a significant decrease in the CD4– subset (P = .02; data not shown). CCR5 expression tended to decrease also in the overall CD4 T-cell compartment (data not shown). This trend reached statistical significance in the ART-only group but not in the ART+IL-2 group (P = .006 and .06, respectively). Less frequent CCR5 expression could contribute to the survival of NKT cells in the early phase of infection, which is typically dominated by R5-tropic HIV-1 strains.19 Moreover, reduced CCR5 surface expression is consistent with a less activated phenotype. Decreased immune activation following IL-2 administration may be critical in sustaining long-lived CD4+ T cell expansions.11 Our data suggest that this may also be the case for NKT cells. Given that general immune activation contributes to HIV-1 pathogenesis,20 the immune-modulating effects of IL-2 could contribute to the normalization of CD4+ T-cell and NKT cell homeostasis. This is interesting, because in some patients T-cell recovery is incomplete despite effective ART.
Our results show that IL-2 therapy given in combination with ART leads to substantial increases in innate CD1d-restricted NKT cell numbers in subjects with primary HIV-1 infection. Because NKT cells are known to be early targets in HIV infection, early therapeutic intervention represents an approach that may maintain and restore the innate NKT cell compartment.
Prepublished online as Blood First Edition Paper, December 20, 2005; DOI 10.1182/blood-2005-09-3636.
Supported in part by grants from The Swedish Foundation for Strategic Research, the Swedish Research Council, the National Institutes of Health (NIH AI52 731 and AI41 531), the Swedish International Development Agency, and Karolinska Institutet. D.F.N. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation. M.M. is a fellow of the European Molecular Biology Organization.
M.M. designed and performed research, analyzed data, and wrote the paper; J.S.-C. designed and performed research; G.S. and F.M.H. provided patient material and analyzed data; J.K.S. designed and performed research and analyzed data; D.F.N. designed research; and all authors checked the final version of the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal