Comment on Rauova et al, page 2346
Rauova and colleagues demonstrate an important role for platelet-associated PF4 levels in modulating the pathogenic potential of anti-PF4/heparin antibodies, even in the absence of pharmacologic heparin.
For reasons that are not clear, only a minority of heparin-treated patients who form anti–platelet factor 4 (PF4)/heparin antibodies develop thrombocytopenia, thrombosis, or other sequelae of heparin-induced thrombocytopenia (HIT). To date, investigators have focused on explaining this clinical heterogeneity through serologic differences, such as antibody class, titer, and platelet-activating properties.1,2 But there are still patients with otherwise typical “HIT antibodies” who do not develop HIT.
In this issue of Blood, Rauova and colleagues examine the role of platelet-associated PF4 in modulating HIT antibody pathogenicity. They have found that a HIT-mimicking anti-PF4/heparin monoclonal antibody (named KKO) exhibited greatly increased binding to human platelets in the presence of added PF4 (maximal binding at 50 μg/mL), even when heparin was not present. Similar findings were observed using purified immunoglobulin G (IgG) obtained from patients with HIT. Moreover, treatment of platelets with chondroitinase, but not heparanase, greatly reduced antibody binding. Thus, HIT antigens are formed by stoichiometrically optimal concentrations of PF4 and platelet surface chondroitin sulfate.
These observations help explain certain features of HIT, such as thrombocytopenia that persists for several weeks or that begins a few days after stopping heparin (“delayed-onset HIT”).3 These data might also help account for why thrombotic risk remains high for a time despite stopping heparin4 (if no alternative anticoagulant is given), perhaps because of continuing platelet activation. The results could also explain the common serologic finding of heparin-independent platelet activation.3,5 FIG1
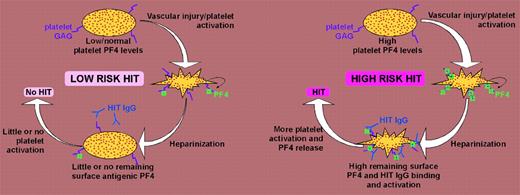
Schematic representation of HIT model. (Left panel) Low or normal platelet-associated PF4 levels result in low expression of HIT antigens on platelet surfaces, with resulting low potential for platelet activation in presence of HIT antibodies. (Right panel) In contrast, high platelet-associated PF4 levels lead to increased expression of HIT antigens on platelet surfaces, predisposing to greater HIT antibody-induced platelet activation and hence greater risk of HIT.
Schematic representation of HIT model. (Left panel) Low or normal platelet-associated PF4 levels result in low expression of HIT antigens on platelet surfaces, with resulting low potential for platelet activation in presence of HIT antibodies. (Right panel) In contrast, high platelet-associated PF4 levels lead to increased expression of HIT antigens on platelet surfaces, predisposing to greater HIT antibody-induced platelet activation and hence greater risk of HIT.
Rauova and colleagues further found that the effects of pharmacologic heparin on binding of KKO to platelets differ depending upon the amount of PF4. When relatively low PF4 concentrations were present (12.5 and 50 μg/mL), added heparin led to reduced antibody binding. In contrast, when high PF4 concentrations were present (200 μg/mL), addition of heparin increased antibody binding to platelets. These data indicate that patient-dependent differences in platelet-associated PF4 quantity could significantly influence risk of HIT (see figure).
The authors further tested these concepts using a murine model of HIT in which various mouse lines expressing human FcγIIA receptors (FcγRIIA) and differing levels of human PF4 (hPF4) were evaluated for thrombocytopenia after intraperitoneal injection with KKO. Whereas FcγRIIA/hPF4high double-transgenic mice developed severe and persisting thrombocytopenia, FcγRIIA/hPF4low mice exhibited only mild, transient platelet count reduction. Pretreatment with protamine sulfate—a small positively charged molecule familiar to clinicians for its heparin-neutralizing properties—prevented thrombocytopenia in both FcγRIIA/hPF4low and FcγRIIA/hPF4high double-transgenic mice, presumably because protamine competes with PF4 for binding to platelet surface chondroitin sulfate. These intriguing results suggest that protamine should be evaluated in clinical studies for its potential to reduce HIT antibody–induced platelet activation. The work of Rauova and colleagues will no doubt stimulate further investigations into the role of platelet-associated PF4 in influencing the clinical expression of HIT. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal