Comment on Berger et al, page 2294
In this issue of Blood, Berger and colleagues show that transducing adoptively transferred T cells with a suicide gene that should only be used in case of toxicity, leads instead to involuntary eradication of the transduced cells by a broad CD4 and CD8 immune response directed to the transgene products.
Adoptive transfer of donor T cells has many potential applications after allogeneic stem cell transplantation, and is used to treat relapsed malignancy and viral infections. Unfortunately, alloreactive T lymphocytes in the infused population can proliferate in vivo, and since these cells may persist for months or years, the resulting graft-versus-host disease can be highly problematic. A suicide gene that can be used in vivo to destroy unwanted donor T cells would therefore increase the safety and broaden the application of this immunotherapy. Several systems have been used to introduce prodrug-metabolizing genes into T lymphocytes so that the cells are killed when the appropriate agent is administered. Initial studies focused on the thymidine kinase gene (TK), which is derived from herpes simplex virus and renders transduced cells sensitive to prodrugs such as ganciclovir. There have been conflicting data on the value of this approach. An initial report in patients with HIV revealed a major limitation of this strategy with the development of a cytotoxic T lymphocyte (CTL) response specific for the TK transgene.1 Subsequent studies of TK in allogeneic transplant recipients have shown variable results, with some investigators finding that the system works as designed, while others demonstrated only short-term persistence.2,3
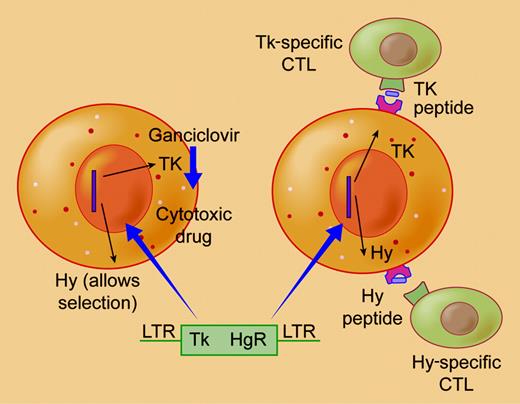
In this issue of Blood, Berger and colleagues report on 3 allogeneic transplant recipients who received donor T cells as therapy for relapse. The cells were transduced with a construct encoding TK and also hygromycin (HY), used as a selectable marker. Both transgenes produced an immune response and the authors' detailed analysis identified multiple epitopes as the immune targets (see figure). Since multiple epitopes could be recognized, modification to remove immunogenic sequences will not be possible, and as the Tk-Hy–specific immune response contains memory cells, it will persist long term. Although the results differ from those of the Milan group who used a vector encoding TK and the nerve growth factor receptor (NGFR),2 the current study confirms that both Tk and Hy are potentially immunogenic even in immunosuppressed patients after transplantation, and emphasizes the need for nonimmunogenic suicide genes.FIG1
The rationale of the suicide gene approach is that cells transduced with the construct encoding a hygromycin phosphotransferase (Hy) thymidine kinase (TK) fusion gene can be selected on the basis of hygromycin resistance and then eradicated if adverse effects occur by administration of gangiclovir which will be converted by Tk to a cytotoxic agent (left). However, the viral-derived Tk product and bacterial-derived Hy products proved immunogenic presenting multiple peptides that could generate a cytotoxic T cell response that effectively eradicated transduced cells (right). Illustration by Frank Forney.
The rationale of the suicide gene approach is that cells transduced with the construct encoding a hygromycin phosphotransferase (Hy) thymidine kinase (TK) fusion gene can be selected on the basis of hygromycin resistance and then eradicated if adverse effects occur by administration of gangiclovir which will be converted by Tk to a cytotoxic agent (left). However, the viral-derived Tk product and bacterial-derived Hy products proved immunogenic presenting multiple peptides that could generate a cytotoxic T cell response that effectively eradicated transduced cells (right). Illustration by Frank Forney.
One means of attaining such an end may be to develop a suicide strategy which uses human rather than gene products derived from bacteria or viruses. A strategy relying on novel artificial death switches based on chemical inducers of dimerization (CIDs) and endogenous proapoptotic molecules has been described.4,5 In this approach, human apoptosis molecules can be linked to FK506 binding proteins that contain a binding site for a CID. Administration of this drug then results in the formation of a complex of 2 apoptosis-fusion molecules, which leads to their activation and thus apoptosis. Recent reports show that both fas and caspase 9 molecules can be used in this way, although caspase 9 may kill a higher percentage of transduced cells, including those that have unregulated inhibitors of the apoptosis pathway.4,5 With such all-human systems, suicide may become a directed rather than an involuntary phenomenon. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal