The human/sheep xenograft model has proven valuable in assessing the in vivo hematopoietic activity of stem cells from a variety of fetal and postnatal human sources. CD34+/lineage- or CD34+/CD38- cells isolated from human embryonic stem cells (hESCs) differentiated on S17 feeder layer were transplanted by intraperitoneal injections into fetal sheep. Chimerism in primary transplants was established with polymerase chain reaction (PCR) and flow cytometry of bone marrow and peripheral blood samples. Whole bone marrow cells harvested from a primary recipient were transplanted into a secondary recipient. Chimerism was established as described before. This animal was stimulated with human GM-CSF, and an increase in human hematopoietic activity was noted by flow cytometry. Bone marrow aspirations cultured in methylcellulose generated colonies identified by PCR to be of human origin. We therefore conclude that hESCs are capable of generating hematopoietic cells that engraft primary recipients. These cells also fulfill the criteria for long-term engrafting hematopoietic stem cells as demonstrated by engraftment and differentiation in the secondary recipient.
Introduction
Human embryonic stem cell (hESC) lines have been highlighted as having the potential to serve as a reservoir for stem cells of diverse lineages.1 Various methods have already been established for their differentiation into hematopoietic cells. These include coculturing on mouse bone marrow stromal cell line S17 or on the yolk sac endothelial cell line C166,2 on S17 with cytokines and bone morphogenetic proteins (BMPs),3 and on OP9 mouse bone marrow stromal cell line4 and forming embryoid bodies in the presence of cytokines and BMP-4.5 While hematopoietic precursor or progenitor cells typified by the CD34 surface marker have been manipulated in vitro to demonstrate their hematopoietic colony-forming ability, thus far a true demonstration of hematopoietic stem cells (HSCs) capable of long-term engraftment and differentiation in a primary followed by a secondary recipient has been lacking.
One challenge for the engraftment of cells from an embryonic source is to find an age-matched recipient. It has been demonstrated in mice that the ability to reconstitute lethally irradiated adults fails when HSCs, harvested further back in development from the yolk sac, are transplanted.6 However, these embryonic cells do contribute to adult hematopoiesis if transplanted into newborn pups where the liver is still the major hematopoietic site. Further, the transplanted embryonic cells in newborn pups can reconstitute conditioned adults upon aging in the primary hosts. The fetal liver of pups was hypothesized to provide a developmentally matched microenvironment for the maturation of naive HSCs into definitive progenitors. In the present studies we derive HSCs from hESCs and carry out transplantations in utero into fetal sheep at the start of the second trimester of gestation, with the notion that it provides an age-relevant microenvironment.
Study design
hESC line H1 (Wicell, Madison, WI) was derived and maintained as described.1 Cells passaged 35 to 56 times were used in this study. Differentiation along the hematopoietic lineage carried out on mouse bone marrow stromal cell line S17 (kindly provided by Dr Kenneth Dorshkind, UCLA) yielded hematopoietic elements as previously characterized.2 Cultures were started in Madison and shipped to Reno overnight after around 8 to 14 days of differentiation in flasks that were topped with media. On day 17, cells were harvested2 and CD34+ cells concentrated with magnetic anti-CD34 microbeads (Miltenyi, Auburn, CA). CD34+/lineage- (CD34+/Lin-) or CD34+/CD38- cells were further purified on FACS Vantage (Becton Dickinson, Mountain View, CA).7 The lineage cocktail included CD2, CD4, CD8, CD14, CD15, CD16, CD19, and glycophorin A.
Seventeen preimmune Dorset Merino sheep fetuses (< 65 days old, term gestation is 150 days) received transplants of 13 000 to 140 000 CD34+/Lin- or CD34+/CD38- cells derived from hESCs. Cells were injected in utero into the fetal peritoneal cavity.8 Lambs were born at term 3 months later. DNA was prepared from bone marrow (BM) and peripheral blood (PB) samples, and polymerase chain reaction (PCR) specific for the human microsatellite DNA was carried out (Supplemental Methods, available on the Blood website; see the Supplemental Materials link at the top of the online article). Animals were stimulated with GM-CSF (sargramostim; Berlex, Montville, NJ) at 5 μg/kg/d for 5 days, and flow cytometry for human hematopoietic elements in BM and PB was carried out before and after GM-CSF treatment using human-specific antibodies and 1 μg/mL 7AAD (7-amino-actinomysin D) for viability gating of cells. Sheep cells do not respond to human-specific GM-CSF.
One animal (no. 1757) that was positive by PCR of BM (Figure S1) and responded to GM-CSF was killed at 1 year after transplantation and its BM cells (0.2% human CD34 by flow cytometry) were cryopreserved in DMSO (Invitrogen, Carlsbad, CA). One million total BM cells were later injected into a secondary fetal sheep recipient as previously described.8 This animal (no. 2026) was tested for chimerism as described. In addition, BM aspirations from the iliac bone were cultured in methylcellulose with cytokines and the resulting colonies were checked for human DNA with PCR for the human beta-2–microglobulin gene.9
Results and discussion
Seventeen animals received injections of 13 000 to 140 000 CD34+/Lin- or CD34+/CD38- cells. The cells used were analyzed by reverse transcriptase–PCR (RT-PCR) for the presence of stem cell markers, and Nanog was absent whereas Oct4 was present (Figure S2). Nine animals were killed at 3 to 29 months after transplantation and their general physiology inspected; they showed no signs of teratomas. Recent data with nonhuman primate ES cells suggest that the sheep model does not support primate teratoma formation.10 Eight live primary recipients were tested for chimerism in BM and PB by PCR and flow cytometry (Table 1). Generally, chimerism by flow cytometry was first noticed at 2 months after birth even though PB from week-old lambs was analyzed. Data reported is for flow cytometry performed at 5 to 17 months after transplantation. Typically there were 0.1% CD34 or CD45 human cells in BM and/or PB although up to 0.5% was seen and 1% to 2% of other markers could be demonstrated in some animals, especially after GM-CSF stimulation. While 0.1% represents only low-level engraftment, this does represent a significant number of cells: a year-old lamb is estimated to contain 1.5 kg of BM and 1 mg is equivalent to one million cells. In comparison, it has previously been shown with 67 000 human cord blood CD34+ cells that 2% to 3% chimerism was typical.11
Chimerism in primary sheep that received transplants of hESC-derived HSCs
. | . | . | . | Chimerism in BM by PCR, %*(time, mo) . | Chimerism by FACS, % (time, mo)† . | . | |
|---|---|---|---|---|---|---|---|
| Animal no. . | No. H1 passages . | Cell type . | No. cells . | . | BM . | PB . | |
| 1748 | 56 | CD34+/Lin- | 140 000 | 0.05 (39) | 0.1 (10) | 0.1 (10) | |
| 1749 | 56 | CD34+/Lin- | 140 000 | 0 (39) | 0.1 (10) | 0.1 (10) | |
| 1757 | 56 | CD34+/Lin- | 100 000 | +‡ (6) | 0 (5) | 0.1 (5) | |
| 1838 | 35 | CD34+/CD38- | 95 000 | 0.04 (35) | 0 (7) | 0 (7) | |
| 1871 | 45 | CD34+/CD38- | 26 500 | 0 (34) | 0 (6) | 0 (6) | |
| 1885 | 48 | CD34+/CD38- | 63 000 | 0.09 (33) | 0.1 (17) | 0.2 (17) | |
| 1886 | 49 | CD34+/CD38- | 70 000 | 0.001 (33) | 0.1 (17) | 0.1 (17) | |
| 1887 | 49 | CD34+/CD38- | 70 000 | 0.001 (33) | 0 (17) | 0.1 (17) | |
. | . | . | . | Chimerism in BM by PCR, %*(time, mo) . | Chimerism by FACS, % (time, mo)† . | . | |
|---|---|---|---|---|---|---|---|
| Animal no. . | No. H1 passages . | Cell type . | No. cells . | . | BM . | PB . | |
| 1748 | 56 | CD34+/Lin- | 140 000 | 0.05 (39) | 0.1 (10) | 0.1 (10) | |
| 1749 | 56 | CD34+/Lin- | 140 000 | 0 (39) | 0.1 (10) | 0.1 (10) | |
| 1757 | 56 | CD34+/Lin- | 100 000 | +‡ (6) | 0 (5) | 0.1 (5) | |
| 1838 | 35 | CD34+/CD38- | 95 000 | 0.04 (35) | 0 (7) | 0 (7) | |
| 1871 | 45 | CD34+/CD38- | 26 500 | 0 (34) | 0 (6) | 0 (6) | |
| 1885 | 48 | CD34+/CD38- | 63 000 | 0.09 (33) | 0.1 (17) | 0.2 (17) | |
| 1886 | 49 | CD34+/CD38- | 70 000 | 0.001 (33) | 0.1 (17) | 0.1 (17) | |
| 1887 | 49 | CD34+/CD38- | 70 000 | 0.001 (33) | 0 (17) | 0.1 (17) | |
FACS indicates fluorescence-activated cell sorter.
Real-time quantitative PCR was carried out for human microsatellite DNA (Supplemental Methods) on BM samples at various periods after transplantation. PCR data for PB samples at the matching time points were all negative. Analyses were repeated at least once to confirm data reported. Minimal detection limit = 0.0001%
Flow cytometry analyses for human CD34 or CD45 was carried out at various periods after transplantation. Twenty thousand to 50 000 events were acquired for a single analysis per animal at the noted time point
Animal no. 1757 was analyzed by PCR for human GAPDH (Figure S1)
PB samples were negative for human DNA by PCR for all primary transplants at the age tested (33-39 months after transplantation) whereas no. 1757 was not tested (Table 1). BM samples were chimeric for 6 of 8 animals tested from 0.001% to 0.09% with a detection limit of 0.0001%. One animal (no. 1749) was positive by flow cytometry at 10 months but negative by PCR at 39 months. As the recipient animals grow large, the engrafted human cells are typically more diluted (Table S1). Therefore the reduction of percentage of human cells seen here may result from the lapse in time when PCR was performed compared with when flow cytometry was performed. One animal (no. 1871) showed lack of engraftment by PCR and flow cytometry.
Others have derived the gene expression profile of the CD34+/CD38- progeny of hESCs (passage 29) and correlated it to HSCs from an early developmental stage, at which time the yolk sac and fetal liver are the primary sites of hematopoiesis.3 In mice, HSCs derived from the yolk sac or mouse ESCs could engraft age-mismatched adult recipients, provided the Hoxb4 gene was engineered for expression in the HSCs.12 Hoxb4 conferred definitive hematopoietic capacity on mouse primitive hematopoietic precursors, but whether it functions the same way during human hematopoietic, development is not known. The fetal sheep model was imperative for the success of our assay for unmodified HSCs differentiated from hESCs in providing donor cells with a developmental age–matched recipient microenvironment. This model has also been used to show engraftment of macaque ESC-derived hematopoietic progeny in primary recipients where it was estimated that 4 × 107 differentiated, nonsorted cells were necessary to achieve 1% chimerism.10
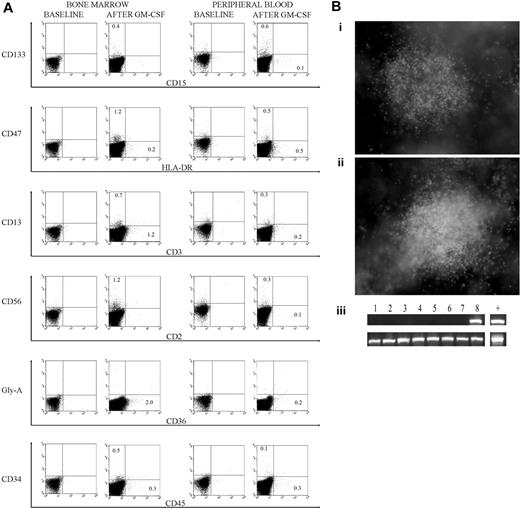
Since sheep hematopoietic cells do not respond to human GM-CSF, donor-specific hematopoiesis could be obtained with a human-specific GM-CSF stimulation. The response from the secondary recipient was comparable to that from primary recipients; data from the secondary recipient is shown at 13 months after transplantation for before and after GM-CSF treatment (Figure 1A) and control data are included (Figure S3). The post–GM-CSF data confirmed differentiation of hematopoietic elements into both myeloid (CD15, CD36) and lymphoid-like (CD2) lineages.13,14 We also show HLA-DR, a major histocompatibility complex (MHC) class II marker for antigen-presenting cells, to be expressed in vivo as has been noted in vitro for hematopoietic progeny derived from hESCs.15 MHC II molecules are decreased on most erythroid and myeloid differentiated cells.16,17 Human CD4 and CD8 are absent in the sheep model. Interestingly, CD133 was expressed higher than CD34 or CD45 in PB but its significance is not known, as this antigen can also be expressed on undifferentiated hESCs,2 on circulating endothelial progenitor cells,18 and on HSCs.19 CD19 was not detected (data not shown). That CD45 is seen at a lower level than other surface markers and is not the sum of other surface antigens can be explained with a down-regulation of its expression in the sheep model.
Flow cytometry data and methylcellulose culture. (A) Flow cytometry data from a secondary sheep, at 13 months after transplantation, that received a transplant of 1 × 106 bone marrow cells harvested from a primary sheep that received a transplant of 100 000 CD34+/Lin- cells derived from hESCs. Samples were collected before and 4 days after a GM-CSF treatment at 5 μg/kg/d for 5 days. (B) Methylcellulose culture of bone marrow cells from a secondary sheep that received a transplant of 1 × 106 bone marrow cells harvested from a primary sheep that received a transplant of 100 000 CD34+/Lin- cells derived from hESCs. Cultures were grown in media including GM-CSF and erythropoietin, and (i-ii) 2 typical granulocyte macrophage colony-forming unit (CFU-GM) colonies are depicted on day 14 (original magnification, × 40). Images were acquired using an Olympus IX71 microscope (Olympus, Melville, NY) with an Olympus Plan 4 × 0.01 numeric aperture objective lens, an Olympus DP70 camera, and Olympus DP Controller 2.1.1.183 software. Images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA). (iii) Colonies were tested for human DNA (PCR for human beta-2–microglobulin) before and 3 days after a 5-day GM-CSF stimulation at 5 μg/kg/d. Three of 24 and 12 of 40 colonies tested positive for human DNA before and after the GM-CSF treatment, respectively. (Top) Human beta-2–microglobulin. (Bottom) Beta actin common to sheep and humans. Human DNA is shown in the last lane and methylcellulose colonies in lanes 1 to 8.
Flow cytometry data and methylcellulose culture. (A) Flow cytometry data from a secondary sheep, at 13 months after transplantation, that received a transplant of 1 × 106 bone marrow cells harvested from a primary sheep that received a transplant of 100 000 CD34+/Lin- cells derived from hESCs. Samples were collected before and 4 days after a GM-CSF treatment at 5 μg/kg/d for 5 days. (B) Methylcellulose culture of bone marrow cells from a secondary sheep that received a transplant of 1 × 106 bone marrow cells harvested from a primary sheep that received a transplant of 100 000 CD34+/Lin- cells derived from hESCs. Cultures were grown in media including GM-CSF and erythropoietin, and (i-ii) 2 typical granulocyte macrophage colony-forming unit (CFU-GM) colonies are depicted on day 14 (original magnification, × 40). Images were acquired using an Olympus IX71 microscope (Olympus, Melville, NY) with an Olympus Plan 4 × 0.01 numeric aperture objective lens, an Olympus DP70 camera, and Olympus DP Controller 2.1.1.183 software. Images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA). (iii) Colonies were tested for human DNA (PCR for human beta-2–microglobulin) before and 3 days after a 5-day GM-CSF stimulation at 5 μg/kg/d. Three of 24 and 12 of 40 colonies tested positive for human DNA before and after the GM-CSF treatment, respectively. (Top) Human beta-2–microglobulin. (Bottom) Beta actin common to sheep and humans. Human DNA is shown in the last lane and methylcellulose colonies in lanes 1 to 8.
Engraftment was confirmed in the secondary recipient (no. 2026) at 16 months after transplantation by PCR for human DNA in both BM (0.0004%) and PB (0.0002%) samples (Table S1). At 25 months, both PB and BM samples were negative. At 26 months, after administering GM-CSF (5 μg/kg/d for 5 days), BM sampled from both hips was positive (0.0002% and 0.0004%) whereas PB remained negative. As mentioned previously, human cells become more obscure with age as the animal gains weight.
Methylcellulose cultures of BM cells from the secondary recipient generated myeloid colonies and 3 of 24 tested positive for human DNA, confirming the presence of human hematopoietic progenitors (Figure 1Bi-iii). This analysis repeated after a human-specific GM-CSF stimulation generated 12 human colonies out of 40 tested (Figure S4). GM-CSF treatment at 13 months after transplantation demonstrated a much higher level of progenitors (30%) than of the 0.1% to 1.2% differentiated hematopoietic elements (Figure 1A). The sheep microenvironment provided a niche for the maintenance of progenitors that proliferated with GM-CSF, whereas differentiation was more limited due to the absence of required human cytokines.
Hence, we have shown engraftment and differentiation of HSCs in the secondary recipient by PCR of BM and PB, flow cytometry after GM-CSF stimulation, and hematopoietic colonies in methylcellulose culture of BM cells. Human hematopoiesis was followed up to 22 months after transplantation by flow cytometry, thus demonstrating the engraftment of stem cells and not the mere transfer of hematopoietic progenitors. It is also of significance to note that HSCs were derived from hESCs that were passaged up to 56 times. Clearly, the number of passages did not exhaust the differentiative and engraftment potential of hESC progeny. Nor did the long (17 day) differentiation period in 20% serum diminish hematopoietic engraftment potential. Others have shown that hESCs that had been passaged numerous times (H1, passage 80) acquired genetic abnormalities.20 However, the low level of engraftment seen in the animals that received a transplant suggests that the human cells did not acquire genetic abnormalities that gave them proliferative advantage over sheep BM cells. We used the S17 feeder layer coculture method of differentiation, which was the initial one available at the time our studies began. This served to prove the capacity of hESCs to generate true HSCs, but other methods3-5 may yield HSCs with different proliferative/engraftment capacities.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2005-05-1922.
Supported by National Institutes of Health (NIH) grants HL52955, HL49042, and HL66058.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal