Paroxysmal nocturnal hemoglobinuria (PNH) results from the expansion of a hematopoietic clone that is deficient in glycosylphosphatidylinositol-anchored molecules. PNH is characterized by chronic hemolysis with acute exacerbations due to the uncontrolled activity of complement on PNH cells, which lack the inhibitor of homologous complement, CD59. Symptoms include severe fatigue, hemoglobinuria, esophageal spasm, erectile dysfunction, and thrombosis. We report the use of a novel synthetically modified recombinant human CD59, rhCD59-P, a soluble protein that attaches to cell membranes. In vitro treatment of PNH erythrocytes with rhCD59-P resulted in levels of CD59 equivalent to normal erythrocytes and effectively protected erythrocytes from complement-mediated hemolysis. The administration of rhCD59-P to CD1 mice resulted in levels of CD59 on erythrocytes, which protected them from complement-mediated lysis. Thus, rhCD59-P corrects the CD59 deficiency in vitro and can bind to erythrocytes in an in vivo murine model, protecting the cells from the activity of human complement, and represents a potential therapeutic strategy in PNH.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is characterized by intravascular hemolysis, venous thrombosis, and an association with aplastic anemia.1 PNH arises through a somatic mutation of the phosphatidylinositol glycan complementation class A (PIGA) gene in a hematopoietic stem cell and the expansion of the abnormal hematopoietic clone. PIGA encodes a protein involved in the first step of glycosylphosphatidylinositol (GPI) biosynthesis.2 GPI structures anchor a wide variety of proteins to the cell surface via their lipid moiety. Deficiency of GPI-linked proteins from PNH erythrocytes can be complete (PNH type III cells) or partial (PNH type II cells). Type I cells express normal levels of GPI-linked proteins. The terminology derives from different complement lysis sensitivities of the 3 types of PNH erythrocytes, type III being 15 to 25 times more sensitive to complement than normal cells.3 This variability in the severity of the deficiency, as well as in the proportion of the affected cell population, defines the clinical manifestations of the disease.4,5 The increased sensitivity to complement results in chronic hemolysis with acute intravascular hemolytic exacerbations causing symptoms of severe fatigue, dark urine, esophageal spasm, and erectile dysfunction in men. Thrombosis is a major cause of morbidity, and even mortality, in this disease.
Among the many proteins missing from the surface of PNH cells are the complement regulatory proteins CD55 (decay accelerating factor) and CD59 (also known as protectin and membrane inhibitor of reactive lysis).6-8 The increased complement sensitivity of PNH cells leads to hemolysis and thrombosis.9 CD55 inhibits C3 and C5 convertases, whereas CD59 is the sole membrane regulator of membrane attack complex (MAC) assembly. As there are additional inhibitors of C3 convertases in human plasma, bystander activation of complement is suppressed even in the absence of CD55. Inherited deficiencies of CD55 (Inab phenotype) are not associated with the symptoms of PNH, indicating that loss of CD55 is not responsible for the hemolysis in PNH.10-12 In contrast, inherited CD59 deficiency results in intravascular hemolysis, hemoglobinuria, and other features indistinguishable from PNH.13,14 As a result of complement-mediated attack, the survival of PNH erythrocytes in vivo is shortened to about 10% that of normal erythrocytes. Patients with only partially deficient (type II) PNH cells do not suffer from significant hemolysis, indicating that low levels of CD59 are sufficient to control terminal complement activity.
The platelets in PNH patients also lack GPI-anchored proteins. The absence of CD59 renders platelets susceptible to attack by complement. When this occurs, the platelets undergo morphologic changes, which result in the release of vesiculated MAC. These vesicles or microparticles are procoagulant in vitro and are present at significantly elevated levels in patients with PNH. It therefore appears that CD59 deficiency from platelets leads to thrombin generation and increased thrombotic risk.15,16
Blocking complement activation by the therapeutic use of eculizumab, a monoclonal antibody to the fifth component of complement (C5), has been shown to be an effective strategy in preventing the symptoms and transfusion requirements resulting from the hemolysis in PNH,17 confirming its complement-dependent nature. However, systemic and prolonged blockade of complement function may have disadvantages. This is indicated by, firstly, inherited deficiency of terminal complement components predisposing to neisserial infections18,19 and, secondly, that prolonged therapeutic inhibition of terminal complement increases the proportion of PNH erythrocytes, raising the possibility of severe intravascular hemolysis if therapy is interrupted.
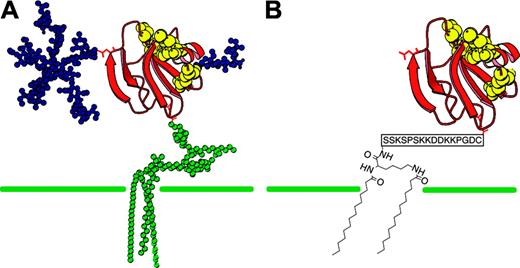
Another approach to the prevention of hemolysis in PNH is to restore CD59 onto the surface of PNH erythrocytes. This should prevent complement-mediated hemolysis and the generation of procoagulant microparticles from platelets while leaving the anti-infective potential of C3 and C5 activation products unaffected. There are a number of practical difficulties associated with using CD59 in its natural GPI-anchored form as a therapeutic agent (see “Discussion”). rhCD59-P is a recombinant human soluble CD59 expressed in Escherichia coli, which has been modified using a C-terminal site-specific attachment of a soluble membrane–interactive peptide to replace the GPI anchor of endogenous CD59 (Prodaptin Technology, Richmond, BC, Canada; Figure 1). The modification enables exogenously administered proteins to bind to the cell membrane in a manner analogous to GPI anchors. A related form of Prodaptin modification has been used previously to localize other complement inhibitors to disease sites in animal models and has been progressed to clinical studies.20-23 Here we report the use of this technology in a new form to enable CD59 replacement therapy in PNH.
Patients, materials, and methods
Protein agents
rhCD59 was prepared by expression in E coli of a synthetic gene encoding residues 1 to 77 of native human CD59 with a C-terminal cysteine residue and refolded from inclusion bodies. The product was reacted with the bis-myristoylated modifying agent APT3146 and purified to yield rhCD59-P as an approximately 9-kDa protein. APT3146 was prepared using conventional peptide synthesis to assemble a sequence KSSKSPSKKDDKKPGDC in which the protecting group strategy enabled both amino groups on the N-terminal lysine to be deprotected and then subjected to N-myristoylation independently of the other lysines. The final thiol-reactive reagent was prepared by reacting the fully deprotected soluble myristoylated peptide with 2,2′ dithiopyridine to yield a C-terminal mixed disulfide. Further details of the design of APT3146 and preparation of rhCD59-P will be presented elsewhere (D.E., I. Dodd, P. J. E. Rowling, G.P.S., J. R. Betley, L. Fiedler, L. Affleck, S. J. Fritchley, S.H.R., and R.A.G.S., manuscript in preparation).
CD59 structure. (A) Native GPI-anchored CD59 compared with (B) rhCD59-P structure. The membrane-localizing unit in rhCD59-P comprises 2 myristoyl groups linked to a common N-terminal lysine residue in a charged hydrophilic peptide terminating in a cysteine residue. A 2-site interaction of such molecules with the hydrophobic interior of the membrane bilayer and with phospholipid headgroups is proposed to account for the affinity of this structure for outer cell membranes.20 The unit is linked to the C-terminus of recombinant human CD59 through an unpaired cysteine residue in the latter.
CD59 structure. (A) Native GPI-anchored CD59 compared with (B) rhCD59-P structure. The membrane-localizing unit in rhCD59-P comprises 2 myristoyl groups linked to a common N-terminal lysine residue in a charged hydrophilic peptide terminating in a cysteine residue. A 2-site interaction of such molecules with the hydrophobic interior of the membrane bilayer and with phospholipid headgroups is proposed to account for the affinity of this structure for outer cell membranes.20 The unit is linked to the C-terminus of recombinant human CD59 through an unpaired cysteine residue in the latter.
Immunofluorescence
Guinea pig blood (10 μL; supplied in Alsever anticoagulant; TCS Biosciences, Botolph Claydon, United Kingdom) was washed twice with 1 mL PBS/0.1% (wt/vol) gelatin. Erythrocytes were resuspended in 200 μL PBS/gelatin in the presence or absence of 250 nM rhCD59-P and incubated for 15 minutes at 37°C. Cells were washed twice with PBS/gelatin prior to incubation for 15 minutes at room temperature with mouse anti–human CD59 monoclonal antibody BRIC229 (IGBRL Research Products, Bristol, United Kingdom), which had been conjugated with Cy3 (Amersham Biosciences, Little Chalfont, United Kingdom), and diluted 1:300 in PBS/gelatin. Cells were washed twice with PBS/gelatin and resuspended in 200 μL PBS immediately prior to visualization by fluorescence and light microscopy. A Nikon Eclipse 400 was used with a CFI PanFluor 40 ×/0.75 numeric aperture objective. Digital images were taken using an Orca ER camera (Hamamatsu Photonics, Welwyn Garden City, United Kingdom) with accompanying image acquisition software. Identical exposure and histogram settings were used between parallel samples.
Reactive lysis assay
For the reactive lysis assay, guinea pig blood (supplied in Alsever anticoagulant; TCS Biosciences) was washed in PBS/0.1% (wt/vol) gelatin and the final erythrocyte pellet was diluted to .05 (5%) hematocrit. Fifty microliters of cells were incubated with 50 μL rhCD59-P or rhCD59 at different concentrations for 30 minutes at 37°C. Ten microliters of human C5b6 euglobulin (prepared in-house from acute-phase serum and diluted 1:100 with PBS/gelatin/15 mM EDTA) and 40 μL normal human serum (pooled from several donors and diluted 1:100 with PBS/gelatin/15 mM EDTA) were then added to the samples. The assay plate was incubated for 1 hour at 37°C prior to centrifugation (300g for 3 minutes at ambient temperature). Hemolysis was determined from the absorbance at 410 nm (A410) of sample supernatants.
Ham test
Human AB serum was obtained from the National Blood Transfusion Service of the United Kingdom or from in-house donors. Blood was collected from PNH patient volunteers after obtaining informed consent (with local ethics committee approval) and stored in EDTA for up to one week. Erythrocytes were washed 3 times in PBS/0.1% gelatin (wt/vol) and resuspended to 0.5 (50%) hematocrit. Cells (7.5 μL) were mixed with 7.5 μL rhCD59-P, rhCD59, or vehicle (diluted in heat-inactivated human AB serum) and 67.5 μL AB serum acidified to pH 6.7 to 7.0 with 1:10 volume of 0.2 M HCl. The assay plate was incubated for 1 hour at 37°C prior to centrifugation. Hemolysis was determined from the A410 of sample supernatants.
Flow cytometry
Whole blood from 6 different patients was incubated with either rhCD59-P or rhCD59 at 37°C for 30 minutes. The blood was washed twice with PBS, a 1:100 dilution was made, and 10 μL of this was added to 10 μL of the working dilution of antibody. The samples were analyzed for CD59 binding by flow cytometry. Anti-CD59–PE (Chemicon, Chandler's Ford, United Kingdom) was used at a 1:10 dilution. Repeat experiments used anti-CD59–FITC (Chemicon) at a 1:5 dilution. This was incubated at room temperature for 1 hour and then analyzed using a FACSCalibur flow cytometer (Becton Dickinson Biosciences, San Jose, CA).
In order to look for neutrophil coating with CD59 after incubation with rhCD59-P, whole blood from 3 different patients was incubated with rhCD59-P at 37°C for 30 minutes, washed twice with PBS, and then analyzed by 3-color flow cytometry with anti-CD59–PE, anti-CD66–FITC (DakoCytomation, Ely, United Kingdom), and anti-CD16–PE:Cy5 (HMDS, Leeds, United Kingdom). The cells were incubated with these antibodies at room temperature for 20 minutes in the dark. One milliliter FACS-lyse (Becton Dickinson Biosciences) was added and further incubated at room temperature for 10 minutes in the dark. After washing twice with PBS, the cells were resuspended in 200 μL of CellFix (Becton Dickinson Biosciences) and left to stand for 15 to 20 minutes before analysis using the FACSCalibur flow cytometer.
In order to establish the range of concentrations over which CD59 was detectable on the surface of the erythrocytes after incubation with rhCD59-P, we repeated the first experiment using blood from 6 patients and incubating with rhCD59-P alone at doubling dilutions from 15 500 nM to 15 nM. Two-color flow cytometry, with anti-CD59–FITC and anti-CD55–PE (Chemicon), was used to ensure that erythrocytes were not hemolysed after incubation with high concentrations of rhCD59-P.
Using 1000 nM rhCD59-P we incubated whole blood from patients with PNH for time periods from 1 minute to 4 hours to establish the rate at which the compound bound to PNH erythrocytes. In order to define the duration of efficacy, erythrocytes bound with rhCD59-P after 30 minutes incubation were maintained for 1 to 7 days at 37°C and at 4°C and were analyzed at regular intervals. In addition, rhCD59-P–bound erythrocytes were stored in either patient's plasma or patient's plasma plus 1000 nM rhCD59-P and were analyzed with 2-color flow cytometry using anti-CD59–FITC and anti-CD235a–PE. The samples stored in patient's plasma plus 1000 nM rhCD59-P were additionally analyzed by flow cytometry after washing twice with PBS before the addition of the antibody solution.
In order to assess whether rhCD59-P transferred to cells after the initial binding, whole PNH blood (taken from patients with a large PNH clone) was incubated with 1850 nM rhCD59-P at 37°C for 30 minutes followed by a PBS wash, producing reconstituted cells. Whole PNH blood (at neat, 1:10, and 1:100 concentrations, using PBS as the diluent) and washed PNH blood cells (at neat, 1:10, and 1:100 concentrations, using PBS as the diluent), with or without plasma, were then added to the reconstituted cells. These were incubated at 37°C and samples were taken at several time points between 1 minute and 72 hours and analyzed by flow cytometry.
In a separate experiment to assess whether rhCD59-P was transferred to the supernatant and retained the ability to bind to untreated PNH cells, PNH whole blood was incubated with 1850 nM rhCD59-P at 37°C for 30 minutes, following which the cells were washed and 200 μL PBS was added. The cells were centrifuged at 850g for 1 minute and the supernatant was added to untreated whole PNH blood (neat and 1:10 concentration diluted in PBS). This was incubated at 37°C for 30 minutes and then analyzed by flow cytometry.
In vivo experiments
In order to assess the use of rhCD59-P in vivo, adult female CD1 mice (3 per group) were administered by tail vein injection either vehicle, rhCD59, or rhCD59-P (each at 10 mg/kg or 50 mg/kg and at a volume of 10 mL/kg). Blood samples (50-100 μL) were obtained from tail bleeds into ACD at 15 minutes, 1 hour, 3 hours, 6 hours, and 24 hours, when the mice were terminated. Blood was centrifuged and plasma removed for analysis. The mouse erythrocytes were then subjected to a human complement challenge using diluted serum, which resulted in hemolysis (mediated by the alternative pathway; data not shown). Erythrocyte pellets were washed once with PBS/0.1% (wt/vol) gelatin and reconstituted to an average of approximately .25 (25%) hematocrit with PBS/gelatin. Five microliters of erythrocytes were mixed with either 45 μL normal human AB serum (National Blood Transfusion Service of the United Kingdom; and diluted 1:8 with PBS/gelatin) or 45 μL PBS/gelatin alone. An additional 5 μL of erythrocytes was mixed with 200 μL water to provide an Amax. The assay samples were then incubated at 37°C for 1 hour prior to centrifugation. Hemolysis was determined from the A410 of sample supernatants. This value was divided by the Amax for each sample to correct for any small differences in cell numbers between samples. The corrected values for the blood samples from vehicle-treated mice were set at “100% hemolysis” for each time point. The values from compound-treated mice were then expressed as percent hemolysis relative to the corresponding 100% value. The experiment was repeated with 2 groups of 5 CD1 mice injected with either vehicle or 50 mg/kg rhCD59-P. Time points for blood sampling were 3 hours, 24 hours, and 48 hours.
In a separate experiment, 2 groups of CD1 mice (3 per group) were injected with either vehicle or 10 mg/kg rhCD59-P. Samples were taken at 1 hour, 4 hours, 24 hours, and 48 hours. The amount of CD59 coating the erythrocytes was assessed by flow cytometry. Each sample was analyzed in triplicate. As a control, mouse erythrocytes were incubated in vitro with doubling dilutions of rhCD59-P from 6200 nM to 775 nM as standards at each time point. Additional in vitro controls included PBS with mouse erythrocytes as a negative control, PBS with human erythrocytes from a healthy volunteer, and PBS with human erythrocytes from a patient with PNH. These samples were run in duplicate. Hemolytic assays were repeated in parallel.
Results
The in vitro activity of rhCD59-P against complement-mediated lysis of guinea pig erythrocytes correlates with cell binding
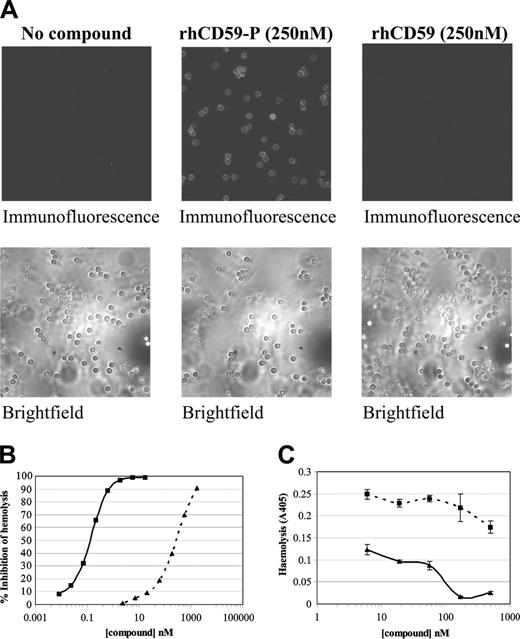
Cell binding activity was determined by in vitro immunofluorescence studies with guinea pig erythrocytes. Binding was demonstrated with rhCD59-P but there was no binding with rhCD59, the parent protein lacking the Prodaptin modification (Figure 2A). This binding correlated with activity in a “reactive lysis” assay24 in which guinea pig erythrocytes were subjected to the activated terminal pathway of human complement. rhCD59-P was found to inhibit hemolysis with a 50% inhibitory concentration (IC50) of approximately 0.2 nM (Figure 2B). In contrast, rhCD59 was over 1000-fold less active in this assay.
In vitro binding of rhCD59-P to erythrocytes and its consequences. (A-B) Guinea pig whole blood was washed and incubated in the presence or absence of either rhCD59-P or the control compound rhCD59 at the concentrations indicated. In panel A, blood cells were then washed and incubated with a fluorescently labeled anti–human CD59 antibody and compound binding was visualized by fluorescence microscopy. Corresponding brightfield images are also shown. In panel B, a hemolytic “reactive lysis” assay was performed on the guinea pig erythrocytes as described in “Reactive lysis assay.” In panel C, a Ham assay was performed on washed whole blood from PNH patient volunteers in the presence or absence of either rhCD59-P (solid line) or rhCD59 (dotted line). Data points show means of direct hemolysis ± SDs of triplicate values. A representative experiment is shown.
In vitro binding of rhCD59-P to erythrocytes and its consequences. (A-B) Guinea pig whole blood was washed and incubated in the presence or absence of either rhCD59-P or the control compound rhCD59 at the concentrations indicated. In panel A, blood cells were then washed and incubated with a fluorescently labeled anti–human CD59 antibody and compound binding was visualized by fluorescence microscopy. Corresponding brightfield images are also shown. In panel B, a hemolytic “reactive lysis” assay was performed on the guinea pig erythrocytes as described in “Reactive lysis assay.” In panel C, a Ham assay was performed on washed whole blood from PNH patient volunteers in the presence or absence of either rhCD59-P (solid line) or rhCD59 (dotted line). Data points show means of direct hemolysis ± SDs of triplicate values. A representative experiment is shown.
rhCD59-P protects PNH erythrocytes in the Ham assay
The Ham test has been widely used in the diagnosis of PNH and is a measure of hemolysis in the presence of acidified human serum.25 rhCD59-P (but not rhCD59) strongly inhibited hemolysis of erythrocytes in this assay (Figure 2C). rhCD59-P was protective in Ham assays using erythrocytes from different PNH patients, with an average IC50 of 150 nM.
In vitro attachment of rhCD59-P to human PNH erythrocytes
The in vitro binding of rhCD59-P to PNH erythrocytes was investigated next for 2 reasons: firstly, to correlate levels of cell surface binding with levels of protection in the Ham assay and, secondly, to use the ability of flow cytometry to detect 3 discrete populations of PNH cells based upon surface levels of CD59.
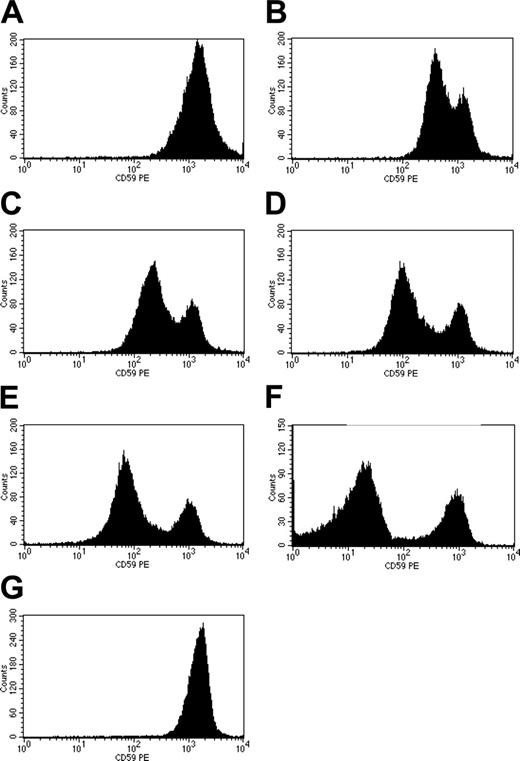
Different concentrations of rhCD59-P were incubated with whole blood from 6 PNH patients and the levels of surface CD59 were compared with non-PNH human red cells analyzed concomitantly. Binding was shown to be dose dependent with normal levels of CD59 on PNH erythrocytes being achieved at high concentrations of rhCD59-P (Figure 3A-G), effectively reversing the CD59 deficiency of the PNH phenotype. Levels of CD59 similar to that found on type II cells were achieved at concentrations of at least 100 nM rhCD59-P in all patient blood samples, but binding of rhCD59-P to erythrocytes was evident below 100 nM. The compound also bound to neutrophils (data not shown). In contrast, no blood cell binding of unmodified rhCD59 was detected when used at 1700 nM (data not shown). Double fluorescent staining for CD55 and CD59 demonstrated that PNH erythrocytes were CD59+ following incubation with rhCD59-P and remained CD55-. Binding onto PNH type III cells was rapid, and the CD59 remained bound on the erythrocytes after 3 days storage at either 4°C or 37°C (data not shown). The CD59 remained detectable, albeit reduced in amount, on the erythrocytes after 24 hours.
Binding of rhCD59-P to PNH erythrocytes. (A-E) Washed whole blood from PNH patients was incubated with rhCD59-P at 15 500 nM (A), 1938 nM (B), 969 nM (C), 484 nM (D), or 121 nM (E). Cells were washed and surface levels of human CD59 were determined by flow cytometry, using a fluorescent anti–human CD59 antibody. Untreated PNH cells are shown in panel F; normal human erythrocytes are shown in panel G.
Binding of rhCD59-P to PNH erythrocytes. (A-E) Washed whole blood from PNH patients was incubated with rhCD59-P at 15 500 nM (A), 1938 nM (B), 969 nM (C), 484 nM (D), or 121 nM (E). Cells were washed and surface levels of human CD59 were determined by flow cytometry, using a fluorescent anti–human CD59 antibody. Untreated PNH cells are shown in panel F; normal human erythrocytes are shown in panel G.
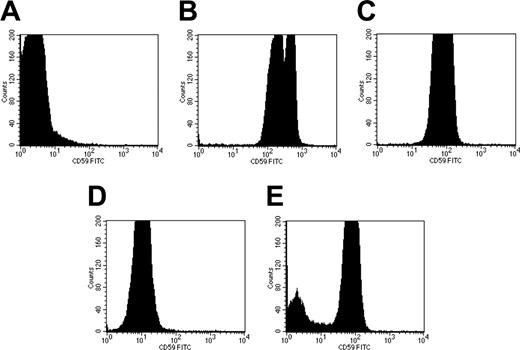
The transfer of rhCD59-P between PNH erythrocytes in vitro. Whole PNH blood (A) was incubated with 1850 nM rhCD59-P for 30 minutes at 37°C, followed by a PBS wash to produce “CD59-reconstituted” cells (B). A separate sample of PNH whole blood was diluted 1:100 in PBS and incubated in the presence of serum with the reconstituted cells at 37°C for either 1 minute (C) or 24 hours (D). As a negative control, non-PNH human blood was incubated with 1:100-diluted PNH whole blood for 30 minutes at 37°C (E). Surface levels of human CD59 were measured on all samples by flow cytometry using a fluorescent anti-CD59 antibody.
The transfer of rhCD59-P between PNH erythrocytes in vitro. Whole PNH blood (A) was incubated with 1850 nM rhCD59-P for 30 minutes at 37°C, followed by a PBS wash to produce “CD59-reconstituted” cells (B). A separate sample of PNH whole blood was diluted 1:100 in PBS and incubated in the presence of serum with the reconstituted cells at 37°C for either 1 minute (C) or 24 hours (D). As a negative control, non-PNH human blood was incubated with 1:100-diluted PNH whole blood for 30 minutes at 37°C (E). Surface levels of human CD59 were measured on all samples by flow cytometry using a fluorescent anti-CD59 antibody.
Transfer of rhCD59-P from reconstituted PNH cells to nonreconstituted PNH cells
To assess whether rhCD59-P could transfer from reconstituted PNH cells (after an initial incubation with 1850 nM rhCD59-P) neat, 1:10, and 1:100 concentrations of whole and washed PNH blood cells were added to the reconstituted cells for different times between 1 minute and 24 hours. The results showed that there was transfer of the protein to the nonreconstituted added cells after incubation at 37°C (Figure 4) and that this transfer was rapid (occurring during the 1-minute time point). Similar levels of coating were found after the addition of 1:10 and 1:100 whole and washed PNH blood cells. The cells remained maximally coated at 3.5 hours (data not shown), with a slight decrease by 24 hours (Figure 4). Adding neat whole PNH blood showed evidence of binding of the protein but at a reduced level compared with the 1:10 and 1:100 dilutions, with lower amounts still when neat washed PNH blood cells were added (data not shown). This may be due to high-density lipoproteins acting as carriers of CD59, as reported by Vakeva et al.26
Ability of supernatant from reconstituted PNH cells to transfer rhCD59-P to untreated PNH cells
To assess whether rhCD59-P was transferred to the supernatant and retained the ability to bind to untreated PNH cells, reconstituted PNH cells (after an initial incubation with 1850 nM rhCD59-P) were washed and PBS was added. After further centrifugation the supernatant was added to neat and 1:10 untreated whole PNH blood (ie, nonreconstituted cells). There was increased binding of CD59 after the addition of 1:10 whole PNH blood (data not shown). This demonstrates the ability of rhCD59-P to dissociate from the red cells into the surrounding fluid, which is then capable of correcting the CD59 deficiency of nearby cells.
Administration of rhCD59-P to CD1 mice results in erythrocyte binding and protection
To assess whether the activities of rhCD59-P shown in vitro could be replicated in an in vivo setting, CD1 mice were injected with the agent and blood samples were taken at time points for analysis by flow cytometry and hemolytic assay.
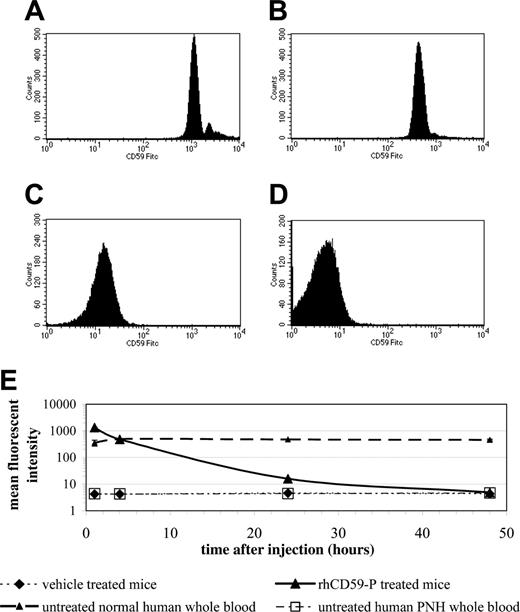
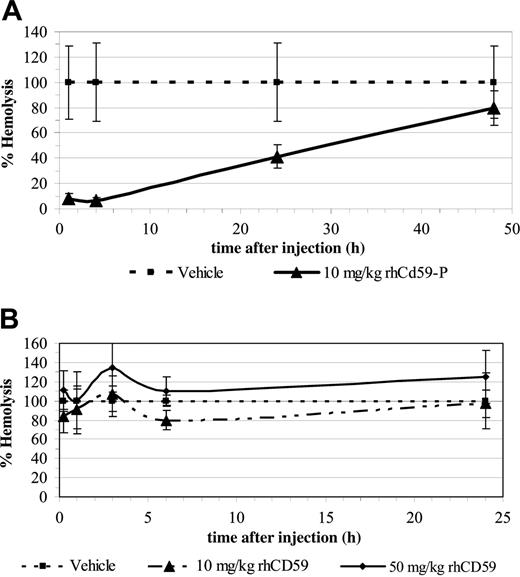
Erythrocytes from mice after intravenous injection of 10 mg/kg rhCD59-P had supranormal levels of CD59 relative to normal human cells at 1 hour after injection, normal levels at 4 hours, and levels equivalent to those found in patients with partially deficient (type II) PNH erythrocytes at 24 hours, with none being detectable at 48 hours (Figure 5A-E). Parallel hemolytic assays using human serum as a complement source revealed that erythrocytes from mice injected with 10 mg/kg rhCD59-P were protected against lysis, at least in part, for up to the 24 hours in comparison with erythrocytes from vehicle-injected mice (Figure 6A). In a separate experiment, 50 mg/kg rhCD59-P afforded full protection against hemolysis at 24 hours (data not shown). In contrast, the control compound rhCD59 demonstrated no protection of erythrocytes at either 10 mg/kg or 50 mg/kg (Figure 6B). An enzyme-linked immunosorbent assay (ELISA)–based comparison of the clearance of rhCD59-P and rhCD59 from the plasma fluid phase showed that the former was significantly more slowly eliminated (half-life ∼ 3 hours versus < 1 hour for unmodified rhCD59; data not shown).
Discussion
The data presented provide a demonstration of the feasibility of CD59 replacement therapy in PNH. Theoretically, this could prevent the considerable morbidity associated with complement-mediated red cell lysis and thrombosis in this group of patients. This work has shown that a form of CD59 modified with a novel synthetic membrane-anchoring peptide efficiently attaches to the surface of PNH erythrocytes in vitro and can deliver CD59 to blood cells at levels that restore complement regulatory activity. It is recognized that even low levels of CD59 on erythrocyte membranes are sufficient to protect the cells from lysis in vitro.
The binding of rhCD59-P to mouse erythrocytes following in vivo administration. CD1 mice (3 per group) were injected with either vehicle or rhCD59-P (10 mg/kg). Blood samples were removed from tail bleeds at time intervals, and surface levels of human CD59 were determined by flow cytometry using a fluorescent anti–human CD59 antibody. Histograms show data from the treated group following 1 hour (A), 4 hours (B), 24 hours (C), and 48 hours (D). The mean fluorescent intensities from each group are summarized in panel E.
The binding of rhCD59-P to mouse erythrocytes following in vivo administration. CD1 mice (3 per group) were injected with either vehicle or rhCD59-P (10 mg/kg). Blood samples were removed from tail bleeds at time intervals, and surface levels of human CD59 were determined by flow cytometry using a fluorescent anti–human CD59 antibody. Histograms show data from the treated group following 1 hour (A), 4 hours (B), 24 hours (C), and 48 hours (D). The mean fluorescent intensities from each group are summarized in panel E.
In vivo administration of rhCD59-P results in the protection of mouse erythrocytes against an ex vivo complement challenge. CD1 mice (3 per group) were injected with either rhCD5-P at 10 mg/kg (A) or the control compound rhCD59 at 10 or 50 mg/kg (B). Blood samples were removed from tail bleeds at the indicated times and were washed and exposed to a human complement challenge as described in “In vivo experiments.” Data points show mean percentage hemolysis at each time point relative to the hemolysis obtained with samples from vehicle-treated mice (taken to represent 100% hemolysis). Error bars represent standard deviations for each group.
In vivo administration of rhCD59-P results in the protection of mouse erythrocytes against an ex vivo complement challenge. CD1 mice (3 per group) were injected with either rhCD5-P at 10 mg/kg (A) or the control compound rhCD59 at 10 or 50 mg/kg (B). Blood samples were removed from tail bleeds at the indicated times and were washed and exposed to a human complement challenge as described in “In vivo experiments.” Data points show mean percentage hemolysis at each time point relative to the hemolysis obtained with samples from vehicle-treated mice (taken to represent 100% hemolysis). Error bars represent standard deviations for each group.
In previous studies, truncated forms of CD59 lacking the GPI anchor have been shown to inhibit complement-mediated lysis of guinea pig erythrocytes in vitro but with approximately 100-fold lower efficiency than the GPI protein. Soluble CD59 lacking the GPI anchor, such as that found in PNH patients, is rapidly removed from the circulation through the kidneys on account of its low molecular weight.27 When isolated with their GPI anchors intact, GPI-linked proteins can readily reinsert into cell membranes and cell-to-cell transfer of such proteins has been demonstrated.28-34 PNH erythrocytes, in vitro, have been shown to acquire resistance to complement-mediated lysis after preincubation with CD59 purified from normal erythrocytes.35 This effect is dependent on the rebinding of the protein to the cell membrane via the GPI anchor. In the presence of serum, the ability of GPI-CD59 to reinsert into the cell membrane of guinea pig erythrocytes is greatly reduced or eliminated. It is likely that this is due to the adsorption of the protein onto serum albumin and/or lipoproteins via the GPI anchor. Thus, quite apart from the problems of large-scale manufacture of GPI-anchored proteins, GPI-CD59 is unlikely to be useful for therapeutic purposes. Rother et al36 used a recombinant transmembrane form of CD59 (CD59-TM) and demonstrated similar levels of protection against human complement–mediated membrane damage with equal levels of either CD59-TM or native CD59 (CD59-GPI). Retroviral transduction of GPI-anchoring–deficient mouse L cells with CD59-TM resulted in surface expression of the protein and rendered these cells resistant to human complement–mediated membrane damage. They suggested that retroviral gene therapy with this molecule could provide a treatment for PNH patients.36 However, in view of the frequent coexistence of bone marrow failure in PNH it has proved extremely difficult to obtain hematopoietic stem cells for gene transfer studies.37 Also, gene therapy still presents a number of, as yet, unresolved clinical challenges.
The activity of CD59 is subject to homologous restriction, and murine CD59 cannot maximally regulate the activity of human complement. No complete animal model of PNH exists, although erythrocytes from transgenic mice mosaic for inactive PIGA showed increased sensitivity to complement ex vivo,38 and knock-out mice lacking CD5939 displayed some PNH pathology such as mild spontaneous intravascular hemolysis. rhCD59-P was not tested in CD59-/- mice because human CD59 is significantly less active against rodent MAC than human.40 Therefore we assessed the activity of rhCD59-P using ex vivo challenge of mouse erythrocytes with human serum as a source of complement, mediated by the alternative pathway. The data presented here are, to our knowledge, the first example of an exogenous CD59 being used in a whole animal to coat erythrocytes protecting them from human complement–mediated attack. This provides a proof of concept for PNH and represents a potential novel therapeutic approach for patients with PNH that does not block the terminal activation of complement and therefore would not carry the risks associated with general complement inhibition. The attachment of rhCD59-P to the red cell membrane was found to be very rapid and the interaction appears to be effective for at least 3 days in vitro. Adding PNH cells after the initial attachment of the protein demonstrated transfer or “painting” onto the nonreconstituted cells, suggesting that newly generated PNH cells would also be protected against complement-mediated lysis. Intravenous injection of rhCD59-P effectively coats the erythrocytes of mice and protects them from lysis by human complement for at least 24 hours for as long as rhCD59-P remained in the circulation. The effective half-life may be longer in humans than in mice, given the generally slower clearance of plasma proteins in man. Additionally, the question of immunogenicity will be addressed by studies with rat CD59-P in rats. Both issues will need to be addressed directly in phase 1 clinical studies.
These studies demonstrate that this type of modification of proteins provides an effective method by which essentially any protein can be effectively localized on the surface of blood cells and they provide further evidence for the potentially wide applicability of this targeted approach. Unlike GPI-CD59, rhCD59-P can be made using a combination of recombinant DNA and chemical modification techniques and, at the doses employed, was well tolerated in mice. rhCD59-P is therefore a candidate for testing in humans.
Prepublished online as Blood First Edition Paper, December 1, 2005; DOI 10.1182/blood-2005-02-0782.
One of the authors (R.A.G.S.) is employed by a company (Inflazyme Pharmaceuticals Ltd) whose potential product was studied in the present work.
A.H. and S.H.R. made equal contributions to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank P. Rowling, M. Steward, J. Atkins, A. Heinrich, G. Dearsley, and B. Lobbezoo who played key roles in the production, modification, and evaluation of CD59 and its modifications. We also thank A. J. Ashcroft for his assistance in obtaining murine samples, M. Wormald for the GPI-CD59 structure, and P. J. Lachmann, A. Davies, and B. P. Morgan for encouragement and assistance in several aspects of this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal