Although the β2-adrenergic receptor (β2AR) is the most extensively characterized G-protein-coupled receptor (GPCR), the effects of β-agonists on T-cell subtype function remain poorly understood. In contrast to studies suggesting lack of β2AR expression on type 2 T cells, we demonstrate that type 2 interleukin-13+ (IL-13+) T cells (CD4+ or CD8+) in human peripheral blood lymphocytes (PBLs) can respond directly to β-agonist, with effects including induction of protein kinase A (PKA) activity and associated inhibition of CD3-stimulated CD25 expression; CD3-stimulated IL-13, interferon-γ (IFN-γ), and IL-2 production; and p38 mitogen-activated protein kinase (MAPK) phosphorylation. PGE2 was more efficacious than β-agonist in activating PKA and inhibiting cytokine production. β-agonist and PGE2 also inhibited phorbol myristate acetate (PMA) + calcimycin-stimulated IFN-γ and IL-2 (but not IL-13) production, suggesting that upstream CD3-initiated signaling is not the sole locus of PKA actions. Differential regulation of PMA-stimulated p38, p42/p44, and NF-κB explained the capacity of PGE2 and β-agonist to inhibit IFN-γ but not IL-13 production. The inhibition of CD3 + CD28-stimulated IL-13 production by both β-agonist and PGE2 was reversed at low agonist concentrations, resulting in enhanced IL-13, but not IFN-γ or IL-2, production. These findings identify direct effects of β2AR activation on T-cell subtypes and suggest a complex role for GPCRs and PKA activity in modulating T-cell functions.
Introduction
The regulation of interferon-γ (IFN-γ) and type 2 cytokine production by T cells is important for development of allergy and acute responses to allergen and for the development of interventions. Inhaled β-agonist therapy is the most common treatment for acute asthmatic attacks. The primary beneficial effect of β-agonists is the relaxation of airway smooth muscle to counter airway constriction induced by histamines, leukotrienes, and other contractile agents released upon allergen binding to mast-cell bound IgE. Based on various established and assumed protein kinase A (PKA)-dependent, inhibitory effects on T cells,1-4 monocytic cells,5 and airway smooth muscle,6 it could be predicted that β-agonists, which are capable of activating PKA through the β2-adrenergic receptor (β2AR), also should provide anti-inflammatory effects in asthmatics. However, several studies indicate that β-agonist therapy may actually worsen inflammation.7-11 Moreover, the β2AR has received extensive attention as a candidate gene (ADRB) for asthma.12-14
The endogenous circulating β-agonist epinephrine has been reported to influence T-cell responses in heart failure patients, who typically have elevated levels of serum epinephrine. Yndestad et al reported that peripheral T cells, but not monocytes, from heart failure patients exhibit increased expression of chemokines, IFN-γ, interleukin-18 (IL-18), and tumor necrosis factor (TNF) superfamily genes.15 Gage et al found that combined beta-blocker and angiotensin converting enzyme inhibitor therapy reduced TNF-α secreted by peripheral blood mononuclear cells (PBMCs) from heart failure patients and lowered the ratio of IFN-γ to IL-10 production as well.16 However, the mechanisms for the modulation of cytokine levels by beta-agonist are not known.
β-agonist activation of the β2AR has been reported to block T-cell receptor (TCR)-mediated signaling to inhibit both cytokine production (IFN-γ and IL-2) and proliferation of T cells.17,18 Among the most controversial aspects of β-agonist effects on T cells are its effects on type 2 T cells. Two studies report a β-agonist-mediated inhibition of IL-4 and IL-13 production in freshly isolated human T cells,19,20 whereas most reports, in various systems, including primary and clonal populations and polyclonal lines of T cells in murine and human systems, claim that β-agonists do not directly affect type 2 T cells.17,18,21 Thus, discrepancies in the literature exist regarding the responsiveness of type 2 T cells to β-agonists. Although 2 studies identified β2AR mRNA in Th2-polarized human T-cell cultures,19,20 no studies to date have demonstrated direct effects of β-agonists on type 2 cells.
PGE2, another activator of PKA, has been shown to have protective effects on allergic inflammation,22 reducing inflammation when exogenously provided to subjects23 and potentiating inflammation when antagonized in an in vivo murine model.24 PGE2 inhibits cellular functions in many immune cells, suppressing IL-12 and TNF-α production by monocytic cells,25,26 cytotoxic potential of natural killer (NK) cells,27,28 and IFN-γ and IL-2 production by T cells.1,20,29-32 The effects of PGE2 on type 2 (IL-4+) T cells1,20,30,32,33 and allergy34,35 are less clear and somewhat controversial. Our searches failed to locate reports regarding direct effects of PGE2 on T-cell-IL-13 production, despite the importance of IL-13 in atopic disorders.36,37
Thus, although both β-agonists and PGE2 signal through Gs-coupled receptors linked to PKA activation, studies to date suggest they have disparate effects on T-cell cytokine production. In the present study we dissect the mechanisms underlying differential regulation of IL-2, IL-13, and IFN-γ production by β-agonists and PGE2 on acute stimulation of human peripheral blood lymphocytes (PBLs), both freshly isolated and cultured short term. These starting populations represent T cells, resting or recently activated, that are poised to respond to stimulation for cytokine production. Herein we establish that type 2 cells express β2ARs capable of modulating numerous T-cell functions and provide mechanistic insight into the roles of Gs-coupled receptor and PKA activation in the regulation of cytokine production in T-cell subsets.
Materials and methods
Isolation and short-term culture of PBLs
Venous blood was obtained from healthy volunteers in accordance with a Wake Forest University Health Sciences Institutional Review Board-approved protocol for these studies. Informed consent was provided according to the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated by standard density gradient centrifugation, and monocytes were removed by adherence to plastics to obtain freshly isolated PBLs. For short-term cultures, PBLs were cultured in RPMI-1640 media supplemented with 5% pooled human plasma. When indicated, PBLs were cultured 6 days with IL-2 (50 U/mL, Hoffman-La Roche; Nutley, NJ) + IL-12-neutralizing mAb (0.5 μg/mL, R&D Systems; Minneapolis, MN), or 14 days with IL-2 + anti-IL-12 mAb + phytohemagglutinin-P (PHA-P) (0.5 μg/mL, Sigma; St Louis, MO), CD28 mAb (2 μg/mL, 9.3, American Type Culture Collection; Manassas, VA). IL-4 (R&D Systems) was used (10 ng/mL) in indicated cultures. CD2-/lo T cells were isolated and cultured as detailed in Loza and Perussia.38 Additional reagents used were isoproterenol (ISO), forskolin, dibutryl-cAMP (db-cAMP) (Sigma); bisindolylmaleimide I (Bis I), H89, PGE2, SB203580, and U0126 (Biomol; Plymouth Meeting, PA); Rp-8-Bromo-2′-O-monobutryl-cAMPs (Rp-Br-MB-cAMPs), and Rp-8-CPT-cAMP (Biolog/Axxora; San Diego, CA).
Analysis of VASP-Ser157 phosphorylation
Cells were stimulated as indicated, formalin fixed, permeabilized with saponin buffer, and analyzed for vasodilator stimulated phosphoprotein (VASP)-phospho-Ser157 (fluorescein isothiocyanate [FITC]-labeled 5C6, NanoTools/Axxora; 2° detection with AlexaFluor [AL] 488-labeled goat anti-mouse IgG; Molecular Probes, Sunnyvale, CA) as described for intracellular cytokine detection in Loza et al.39 For analysis of chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) co-expression, mAb for CRTH2 (AL647-labeled, BD Pharmingen; San Diego, CA) and CD161 (biotinylated B199.2, kindly provided by Dr Bice Perussia, Thomas Jefferson University, Philadelphia, PA; detected with AL546-labeled goat anti-mouse IgG, Molecular Probes) were added at the end of stimulation. CD161 detection was used to exclude NK cells, which also may express CRTH2. In our freshly isolated PBL preparations, granulocytes that may express CRTH2 were absent in the lymphocyte populations analyzed (not shown). For analysis of CD56 co-expression, CD56 (AL647-labeled, Caltag Laboratories; Burlingame, CA) and CD5 (TriColor-labeled, Caltag) mAbs were added during stimulation, but 2° detection of 5C6-FITC mAb was excluded to prevent cross-reaction with the CD5 mAb. Phospho-VASP and CD56 were analyzed in gated CD5+ T cells. Samples were analyzed by flow cytometry on a FACS Calibur (Becton Dickinson; San Jose, CA) and data analyzed on FlowJo for Windows software (TreeStar; Ashland OR). For detection of shift in apparent molecular weight of VASP, after stimulation cells were lysed in sample buffer, then standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting were performed, using VASP mAb (BD Transduction Laboratories; Franklin Lakes, NJ) detected with AL680 goat anti-mouse IgG (Molecular Probes). Fluorescence signal from the immunoblot was analyzed on an Odyssey Infrared detection system (Li-Cor; Lincoln, NE).
Analysis of cytokine production
Cells were stimulated either 5 hours with PMA (2 nM, Sigma), calcimycin (200 ng/mL, A23187, Sigma), IL-2 (100 U/mL), and monensin (5 μg/mL, Sigma, to prevent secretion of cytokines), or 18 hours with IL-2, CD28 mAb (2 μg/mL), and monensin on CD3-coated well (5 μg/mL OKT3, eBioscience; San Diego, CA). After stimulation, cells were labeled with CD3 and CD5 TriColor-labeled mAbs (both from Caltag), formalin fixed, permeabilized with a saponin buffer, and analyzed for intracellular cytokines, as detailed in Loza et al.39 IL-2, IL-13, and IFN-γ were detected with FITC-, phycoerythrin (PE)-, and AL647-labeled mAbs, respectively (Caltag and BD Pharmingen) by flow cytometry analysis.
Analysis of p38, p42/p44, and NF-κB p65 phosphorylation
Cells were stimulated 10 minutes, as indicated, fixed with 5% formalin for 10 minutes at 37°C, then permeabilized with 90% methanol. After washing with phosphate-buffered saline (PBS) + 0.1% gelatin, aliquots of cells were stained with mAbs (all from Cell Signaling Technologies; Beverly, MA) specific for p42/p44-phospho-T202/Y204 (AL647), p38 mitogen-activated protein kinase (MAPK)-phospho-T180/Y182 (AL488), and NF-κB p65-phospho-S536 (detected with AL488-labeled goat anti-rabbit IgG), then analyzed by flow cytometry.
Statistical analyses
Gate settings for flow cytometry analyses and net mean fluorescence intensity (MFI) values were established based on the staining of parallel samples with the relevant isotype control monoclonal antibodies. Because MFI values are arbitrary units unique to a particular, independent experiment, and because of interindividual variability in proportions of cytokine-producing T cells for the control conditions (for fresh PBLs, the ranges for PMA + calcimycin-stimulated T cells were 0.3%-4% IL-13+; 5%-20% IL-2+; 2%-35% IFN-γ+), data were normalized to percent change relative to the control condition in order to perform statistical analyses. Statistical analysis of normalized data was performed using GraphPad Prism 4.03 software (San Diego, CA). Normalized data are reported as mean ± SD. Difference of means were considered significant at P < .05.
Results
VASP-phospho-Ser157 is an indicator of PKA activity in T cells
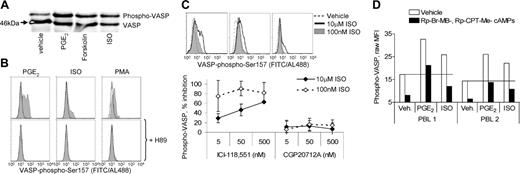
Previous studies have asserted PKA-dependent effects in T cells by either assessing agonist-stimulated PKA activity in an in vitro assay or demonstrating actions of pharmacologic PKA inhibitors and activators.1,29-31,40-43 For this report, we used a more direct approach of analyzing PKA activity in intact cells by detection of phosphorylation of VASP at Ser157, which is mediated directly and selectively by PKA.44 In immunoblot analyses, Ser157 phosphorylation of VASP is reflected in a mobility shift of VASP from 46 kDa to 50 kDa.44 Using this approach, PGE2 and the β-agonist ISO are shown to induce PKA activity in freshly isolated PBLs (Figure 1A). A mAb reactive to VASP-phospho-Ser157 was used to detect VASP-Ser157 phosphorylation by flow cytometry. Both PGE2 and ISO stimulated VASP phosphorylation, although PGE2 was consistently more efficacious than ISO (Figure 1B). ISO-stimulated VASP phosphorylation was inhibited by the β2AR-selective antagonist ICI-118551 but not the β1AR-selective antagonist CGP20712A, suggesting that β-agonists activate predominately, if not exclusively, β2ARs on T cells to induce PKA activity (Figure 1C). Pretreatment of PBLs with the PKA inhibitor H8945 completely inhibited PGE2- and ISO-stimulated VASP phosphorylation (Figure 1B). Although H89 is a potent and highly efficacious PKA inhibitor, interpretation of its effects is confounded by nonspecific actions, including those as a beta-adrenergic receptor antagonist.46 As an alternative approach, we examined the effects of Rp-Br-MB-cAMPs and Rp-8-CPT-cAMPs, which preferentially inhibit activation of type I and type II PKA, respectively.43 In combination, Rp-Br-MB-cAMPs and Rp-8-CPT-cAMPs almost completely inhibited ISO- and PGE2-induced phosphorylation of VASP (Figure 1D).
Multiple agents promote VASP phosphorylation in a PKA-dependent manner. Freshly isolated PBLs were stimulated 30 minutes with vehicle, PGE2 (1 μM), forskolin (50 μM), or ISO (10 μM). (A) Cells were then lysed in sample buffer and subsequently analyzed by SDS-PAGE/immunoblotting for VASP expression. (B) Freshly isolated PBLs were treated 20 minutes with vehicle or H89 (10 μM), followed by a 30-minute stimulation with vehicle, ISO (10 μM), PGE2 (1 μM), or PMA (5 nM). PBLs were then analyzed for expression of VASP-phospho-Ser157 by flow cytometry. Histograms show expression of phospho-VASP (x-axis) in vehicle (top) or H89 (bottom) treated cells (open region) and cells with the indicated treatments (filled region). Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). (C) Freshly isolated PBLs were pretreated 5 minutes with serial dilutions of ICI-118551, stimulated 30 minutes with 100 nM or 1 μM ISO, then analyzed for expression of VASP-phospho-Ser157 by flow cytometry. Histograms of phospho-VASP for ISO-stimulated PBLs are shown (top), and the percent inhibition of ISO-stimulated VASP phosphorylation by antagonist is reported as mean ± SD (bottom; n = 3). (D) Freshly isolated PBLs (n = 2) were treated 10 minutes with vehicle or Rp-Br-MB-cAMPs and Rp-8-CPT-cAMPs (100 μM each), followed by 30-minute stimulation with PGE2 (1 μM) or ISO (10 μM). VASP-phospho-Ser157 expression was analyzed by flow cytometry, and raw MFI values (without background MFI subtracted) are reported.
Multiple agents promote VASP phosphorylation in a PKA-dependent manner. Freshly isolated PBLs were stimulated 30 minutes with vehicle, PGE2 (1 μM), forskolin (50 μM), or ISO (10 μM). (A) Cells were then lysed in sample buffer and subsequently analyzed by SDS-PAGE/immunoblotting for VASP expression. (B) Freshly isolated PBLs were treated 20 minutes with vehicle or H89 (10 μM), followed by a 30-minute stimulation with vehicle, ISO (10 μM), PGE2 (1 μM), or PMA (5 nM). PBLs were then analyzed for expression of VASP-phospho-Ser157 by flow cytometry. Histograms show expression of phospho-VASP (x-axis) in vehicle (top) or H89 (bottom) treated cells (open region) and cells with the indicated treatments (filled region). Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). (C) Freshly isolated PBLs were pretreated 5 minutes with serial dilutions of ICI-118551, stimulated 30 minutes with 100 nM or 1 μM ISO, then analyzed for expression of VASP-phospho-Ser157 by flow cytometry. Histograms of phospho-VASP for ISO-stimulated PBLs are shown (top), and the percent inhibition of ISO-stimulated VASP phosphorylation by antagonist is reported as mean ± SD (bottom; n = 3). (D) Freshly isolated PBLs (n = 2) were treated 10 minutes with vehicle or Rp-Br-MB-cAMPs and Rp-8-CPT-cAMPs (100 μM each), followed by 30-minute stimulation with PGE2 (1 μM) or ISO (10 μM). VASP-phospho-Ser157 expression was analyzed by flow cytometry, and raw MFI values (without background MFI subtracted) are reported.
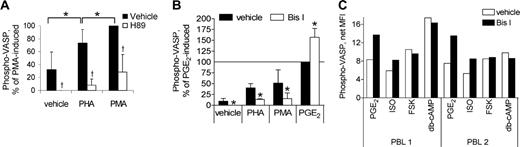
Interestingly, stimulation of either the CD3 complex with PHA or protein kinase C (PKC) with PMA also induced a significant, albeit relatively weak, phosphorylation of VASP, which was inhibited by H89 (Figure 2A). CD28 co-stimulation did not affect PHA-stimulated VASP phosphorylation (not shown). A low level of basal VASP phosphorylation was consistently detected by both immunoblotting (Figure 1A) and flow cytometry and was reversible by H89 (Figure 2A). The VASP phosphorylation induced by both PHA and PMA, as well as the basal level of VASP phosphorylation, also was prevented by Bis I, an inhibitor specific for PKC, indicating that activation of PKA can occur downstream of PKC activation in T cells (Figure 2B). PHA- and PMA-mediated activation of PKA did not result from arachidonate metabolism into prostaglandins, indicated by the lack of effect by the cyclooxygenase inhibitor indomethacin (not shown). Bis I did not inhibit but rather enhanced PGE2-stimulated VASP phosphorylation, suggesting PKC activity serves to desensitize responsiveness to PGE2. Bis I pretreatment also enhanced β-agonist-induced VASP phosphorylation (Figure 2C). The desensitizing effects of PKC likely occurred at the receptor/G-protein locus, as Bis I did not affect VASP phosphorylation by db-cAMP, a direct activator of PKA, or by forskolin, a direct activator of adenylyl cyclase.
Modulation of PKA activity by PKC. (A) Freshly isolated PBLs were stimulated as described in Figure 1B, using PHA (4 μg/mL) and PMA (5 nM) as the stimuli, then analyzed for VASP-phospho-Ser157 by flow cytometry. MFI of phospho-VASP staining was normalized to PMA-induced phospho-VASP = 100% and H89 only-treated cells as 0%, with mean ± SD reported (n = 4). *P < .05 for difference versus PHA-stimulated; †P < .05 for inhibition by H89. (B) Fresh PBLs were treated for 20 minutes with vehicle or Bis I (10 μM), then each was stimulated 30 minutes with vehicle, PMA (2 nM), PHA (4 μg/mL), or PGE2 (1 μM). Cells were then analyzed for VASP-phospho-Ser157 by flow cytometry. MFI of phospho-VASP staining was normalized to PGE2-induced phospho-VASP = 100% and Bis I only-treated cells as 0%, with mean ± SD reported (n = 5). *P < .05 for effect of Bis I. (C) Fresh PBLs were treated 10 minutes with vehicle or Bis I (10 μM), then stimulated 30 minutes with vehicle, PGE2 (1 μM), ISO (10 μM), forskolin (50 μM), or db-cAMP (200 μM). Cells were analyzed for VASP-phospho-Ser157 by flow cytometry, and net MFI values (MFI from vehicle-stimulated condition subtracted) is reported for the 2 PBL samples tested.
Modulation of PKA activity by PKC. (A) Freshly isolated PBLs were stimulated as described in Figure 1B, using PHA (4 μg/mL) and PMA (5 nM) as the stimuli, then analyzed for VASP-phospho-Ser157 by flow cytometry. MFI of phospho-VASP staining was normalized to PMA-induced phospho-VASP = 100% and H89 only-treated cells as 0%, with mean ± SD reported (n = 4). *P < .05 for difference versus PHA-stimulated; †P < .05 for inhibition by H89. (B) Fresh PBLs were treated for 20 minutes with vehicle or Bis I (10 μM), then each was stimulated 30 minutes with vehicle, PMA (2 nM), PHA (4 μg/mL), or PGE2 (1 μM). Cells were then analyzed for VASP-phospho-Ser157 by flow cytometry. MFI of phospho-VASP staining was normalized to PGE2-induced phospho-VASP = 100% and Bis I only-treated cells as 0%, with mean ± SD reported (n = 5). *P < .05 for effect of Bis I. (C) Fresh PBLs were treated 10 minutes with vehicle or Bis I (10 μM), then stimulated 30 minutes with vehicle, PGE2 (1 μM), ISO (10 μM), forskolin (50 μM), or db-cAMP (200 μM). Cells were analyzed for VASP-phospho-Ser157 by flow cytometry, and net MFI values (MFI from vehicle-stimulated condition subtracted) is reported for the 2 PBL samples tested.
Induction of PKA activity in type 2 T cells
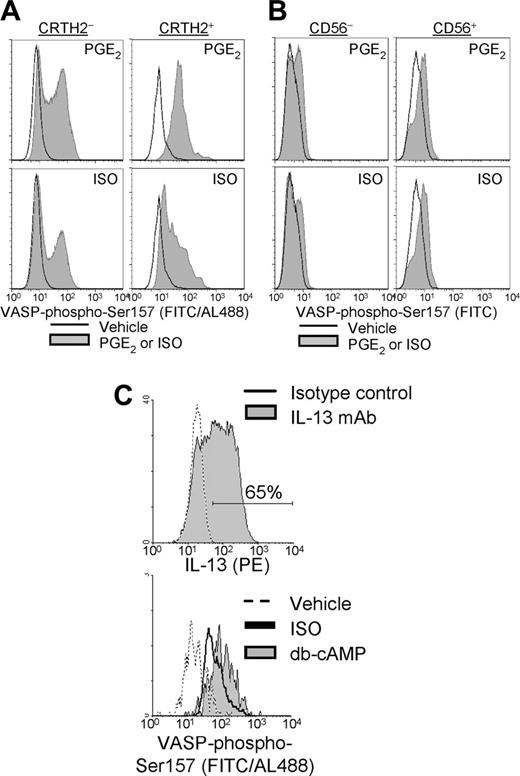
Simultaneous analysis of β-agonist-induced PKA activity and cytokine profile is complicated by the necessary, concomitant stimulation for cytokine production because several hours are required for accumulation of detectable levels of cytokine, the stimulation for cytokine production itself induces PKA activity through PKC, and PKA-activating agents modulate cytokine production (see “Modulation of cytokine production by PKA-activating agonists”). These limitations can be overcome by using surrogate markers (unaffected by stimulation) for the T-cell subsets, namely CRTH2 for type 2 cells and CD56 for type 1 cells. CRTH2+ T cells represent approximately 25% of type 2 T cells, with most (∼ 90%) CRTH2+ cells being IL-13+ and IFN-γ- (not shown; Nagata et al47 ). CD56 is an NK cell differentiation marker that T cells acquire in terminal stages of development into IFN-γ+ cells, in response to IL-12. CD56+ T cells do not express type 2 cytokines, only minimally express IL-2,48 and are CRTH2- (not shown). As shown in Figure 3A, ISO promoted significant Ser157 VASP phosphorylation in CRTH2+ cells, indicating the presence of β2ARs in type 2 cells. PGE2 stimulated even greater levels of phospho-VASP in CRTH2+ cells. In CD56+ cells, both PGE2 and ISO promoted VASP phosphorylation to a similar degree (Figure 3B). To further establish that type 2 T cells directly respond to β-agonist, cultures highly enriched for IL-13+ cells (CD2-/lo T cells, characterized in Loza and Perussia38 ) were tested for ISO-induced VASP phosphorylation. These cells, which were more than 70% IL-13+ (Figure 3C, top) and less than 5% IFN-γ+ (not shown) exhibited profound VASP phosphorylation upon stimulation with ISO (Figure 3C, bottom). Thus, β-agonist-mediated PKA activation is evident in the larger IL-13+ population as well as the CRTH2+ subset of type 2 T cells.
β-agonist-induced PKA activity in type 2 T cells. VASP-Ser157 phosphorylation in CRTH2+ and CRTH2- T cells or CD56+ and CD56- T cells was detected in freshly isolated PBLs after 30 minutes' stimulation with vehicle, PGE2 (1 μM), or ISO (10 μM), as described in “Materials and methods.” VASP-phospho-Ser157 expression is presented in histograms, analyzed in the indicated population within (A) CD161- T cells and (B) CD5+ T cells. Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). Direct comparisons between results in panels A and B cannot be made because amplification of the phospho-VASP signal was only performed in panel A, as explained in “Materials and methods.” (C, top) T cells from CD2-/lo T-cell cultures were stimulated with PMA + calcimycin and analyzed for cytokine production (filled region, fluorescence from IL-13 staining; open region, background fluorescence from isotype control mAb). The preparation was more than 99% CD3+ and less than 5% IFN-γ+ (not shown). (C, bottom) In parallel, VASP-phospho-Ser157 was detected in this T-cell population after 30 minutes' stimulation with vehicle, ISO (10 μM), or db-cAMP (200 μM).
β-agonist-induced PKA activity in type 2 T cells. VASP-Ser157 phosphorylation in CRTH2+ and CRTH2- T cells or CD56+ and CD56- T cells was detected in freshly isolated PBLs after 30 minutes' stimulation with vehicle, PGE2 (1 μM), or ISO (10 μM), as described in “Materials and methods.” VASP-phospho-Ser157 expression is presented in histograms, analyzed in the indicated population within (A) CD161- T cells and (B) CD5+ T cells. Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). Direct comparisons between results in panels A and B cannot be made because amplification of the phospho-VASP signal was only performed in panel A, as explained in “Materials and methods.” (C, top) T cells from CD2-/lo T-cell cultures were stimulated with PMA + calcimycin and analyzed for cytokine production (filled region, fluorescence from IL-13 staining; open region, background fluorescence from isotype control mAb). The preparation was more than 99% CD3+ and less than 5% IFN-γ+ (not shown). (C, bottom) In parallel, VASP-phospho-Ser157 was detected in this T-cell population after 30 minutes' stimulation with vehicle, ISO (10 μM), or db-cAMP (200 μM).
Effects of β-agonist and PGE2 on type 2 T-cell activation
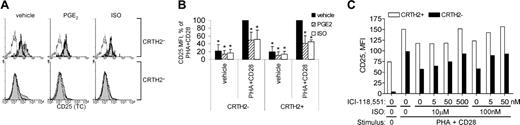
To gain insight into the potential effects of ISO- and PGE2-stimulated PKA activity on type 2 T-cell activation, we examined the effects of these agonists on expression of CD25 (IL-2Rα), a transient activation marker whose expression is up-regulated upon CD3-mediated stimulation. PKA can inhibit CD3-mediated signaling at its most upstream events by inhibiting activation of p56lck via Csk-binding protein (Cbp) and C-terminal src kinase (Csk).2,3 Only a small fraction of the major (CRTH2-) T-cell population in freshly isolated PBLs expressed CD25, whereas most CRTH2+ (type 2) T cells expressed CD25 at moderate levels (Figure 4A; Nagata et al47 ). Stimulation of freshly isolated PBLs with PHA, CD28 mAb, and IL-2 induced up-regulation of CD25 expression in a subset of both CRTH2- and CRTH2+ T cells within 18 hours (Figure 4A). A 30-minute treatment with ISO or PGE2 prior to stimulation significantly reversed this up-regulation in both subsets (Figure 4A,B). The inhibition by ISO was reversed by ICI-118551 (Figure 4C). These results demonstrate direct effects of β-agonist on type 2 T cells via β2AR, consistent with induction of PKA activity.
Modulation of cytokine production by PKA-activating agonists
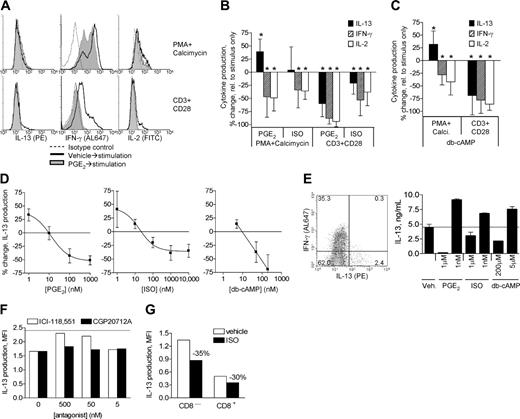
We next examined the effects of PGE2 and ISO on expression of IL-13, IL-2, and IFN-γ in T cells (Figure 5). For our studies, IL-13 is the readout for type 2 T cells because it is the cytokine most broadly expressed by type 2 T cells38,47 and is critical to the pathogenesis of atopic disorders.36,37 In contrast to several reports claiming that β-agonists do not directly affect type 2 (IL-4+) T cells, ISO inhibited CD3-stimulated IL-13 production, though not as effectively as did PGE2. Not surprisingly, PGE2 and ISO inhibited CD3-stimulated IL-2 and IFN-γ production, consistent with previous reports.1,18,20,29-32 The inhibition of IL-13, IFN-γ, and IL-2 production by ISO was less effective than the inhibition by PGE2 for each cytokine. Inhibition of IL-13 production by ISO and PGE2 was less effective than the respective inhibition of IFN-γ and IL-2. The contribution of IL-13+ T cells that co-express IFN-γ was negligible because fewer than 5% of the IL-13+ T-cell populations from freshly isolated T cells and CD2-/lo cultures and fewer than 12% from IL-2- and PHA + CD28 + IL-2-cultured PBLs co-expressed IFN-γ; and the few IFN-γ+ IL-13+ T cells expressed much lower levels of IL-13 than did IFN-γ- IL-13+ T-cell populations (not shown, Figure 5E). The inhibition of IL-13 production by ISO was reversed by ICI-188551 but not by CGP20712A, indicating the inhibition was mediated by β2AR (Figure 5F). ISO inhibited IL-13 production by not only CD4+ (Th2) cells but also by CD8+ type 2 T cells (Figure 5G).
To determine if the PKA-activating agonists can affect signaling important for cytokine production that occurs downstream of the well-characterized block in p56lck activation, cells were stimulated with PMA + calcimycin to bypass the most upstream signaling events involved in CD3-mediated signaling (Figure 5). Surprisingly, under these conditions inhibition of IL-2 and IFN-γ production by PGE2 and ISO remained significant, but the inhibition of IL-13 production was lost. Furthermore, PGE2, but not ISO (variable, with no mean effect), actually augmented PMA + calcimycin-stimulated IL-13 production. Given that the cellular transport inhibitor monensin (present the entire time of stimulation) prevents release of proteins to the cell surface or extracellular medium, paracrine effects from non-type 2 cells to type 2 cells would not account for these results. Similar results were observed when cultures highly enriched for IL-13+ cells (CD2-/lo T cells, Figure 3C) were used, further supporting a lack of contribution of paracrine effects from non-type 2 cells (not shown). CAY10399, a selective EP2 receptor agonist, similarly increased IL-13 production and inhibited IFN-γ and IL-2 production, whereas the selective EP1/EP3 receptor agonist 17-phenyl-trinor-PGE2 did not, suggesting that the effects of PGE2 are mediated mainly by the EP2 receptor (not shown). db-cAMP mimicked the effects of PGE2 in enhancing PMA + calcimycin-induced IL-13 production and attenuating IFN-γ and IL-2 production, implicating cAMP-dependent pathways for the effects of PGE2 and ISO on cytokine production (Figure 5C). The same modulation of cytokine production by PGE2 and ISO was observed in PBLs cultured 6 days with IL-2 (not shown), a condition in which IL-13+ cells preferentially proliferate relative to IL-13- and IFN-γ+ cells.49,50
Inhibition of CD25 induction in type 2 T cells by β-agonist. (A) Freshly isolated PBLs were treated 30 minutes with vehicle, PGE2 (1 μM), or ISO (10 μM), then stimulated 18 hours with vehicle (—) or PHA + CD28 mAb + IL-2 ( ). Cells were simultaneously analyzed for expression of CD3, CRTH2, and CD25 by flow cytometry. - - - indicates isotype control. Expression of CD25 on PHA + CD28 + IL-2-stimulated T cells in CRTH2+ and CRTH2- T cells is presented as histograms. Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). (B) To allow statistical analyses of data, MFI of CD25 for each subject was normalized as the percent of the respective net MFI (background subtracted) in the IL-2 + PHA + CD28-only condition for both subsets. The mean ± SD is shown (n = 5). *P < .05 for difference from 100%. All data sets were significantly less than the respective IL-2 + PHA + CD28 condition (= 100%) (P < .05). (C) Freshly isolated PBLs were incubated with the indicated concentration of the β2AR antagonist ICI-118551 for 10 minutes, treated 30 minutes with the indicated concentration of ISO, and then stimulated 18 hours with PHA + CD28 mAb + IL-2. MFI values of CD25 expression (background subtracted) in gated CRTH2+ (□) and CRTH2 (▪) T cells are reported for 1 representative of 2 independent experiments.
). Cells were simultaneously analyzed for expression of CD3, CRTH2, and CD25 by flow cytometry. - - - indicates isotype control. Expression of CD25 on PHA + CD28 + IL-2-stimulated T cells in CRTH2+ and CRTH2- T cells is presented as histograms. Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). (B) To allow statistical analyses of data, MFI of CD25 for each subject was normalized as the percent of the respective net MFI (background subtracted) in the IL-2 + PHA + CD28-only condition for both subsets. The mean ± SD is shown (n = 5). *P < .05 for difference from 100%. All data sets were significantly less than the respective IL-2 + PHA + CD28 condition (= 100%) (P < .05). (C) Freshly isolated PBLs were incubated with the indicated concentration of the β2AR antagonist ICI-118551 for 10 minutes, treated 30 minutes with the indicated concentration of ISO, and then stimulated 18 hours with PHA + CD28 mAb + IL-2. MFI values of CD25 expression (background subtracted) in gated CRTH2+ (□) and CRTH2 (▪) T cells are reported for 1 representative of 2 independent experiments.
Inhibition of CD25 induction in type 2 T cells by β-agonist. (A) Freshly isolated PBLs were treated 30 minutes with vehicle, PGE2 (1 μM), or ISO (10 μM), then stimulated 18 hours with vehicle (—) or PHA + CD28 mAb + IL-2 ( ). Cells were simultaneously analyzed for expression of CD3, CRTH2, and CD25 by flow cytometry. - - - indicates isotype control. Expression of CD25 on PHA + CD28 + IL-2-stimulated T cells in CRTH2+ and CRTH2- T cells is presented as histograms. Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). (B) To allow statistical analyses of data, MFI of CD25 for each subject was normalized as the percent of the respective net MFI (background subtracted) in the IL-2 + PHA + CD28-only condition for both subsets. The mean ± SD is shown (n = 5). *P < .05 for difference from 100%. All data sets were significantly less than the respective IL-2 + PHA + CD28 condition (= 100%) (P < .05). (C) Freshly isolated PBLs were incubated with the indicated concentration of the β2AR antagonist ICI-118551 for 10 minutes, treated 30 minutes with the indicated concentration of ISO, and then stimulated 18 hours with PHA + CD28 mAb + IL-2. MFI values of CD25 expression (background subtracted) in gated CRTH2+ (□) and CRTH2 (▪) T cells are reported for 1 representative of 2 independent experiments.
). Cells were simultaneously analyzed for expression of CD3, CRTH2, and CD25 by flow cytometry. - - - indicates isotype control. Expression of CD25 on PHA + CD28 + IL-2-stimulated T cells in CRTH2+ and CRTH2- T cells is presented as histograms. Background fluorescence from isotype control mAb was superimposable for all conditions (not shown). (B) To allow statistical analyses of data, MFI of CD25 for each subject was normalized as the percent of the respective net MFI (background subtracted) in the IL-2 + PHA + CD28-only condition for both subsets. The mean ± SD is shown (n = 5). *P < .05 for difference from 100%. All data sets were significantly less than the respective IL-2 + PHA + CD28 condition (= 100%) (P < .05). (C) Freshly isolated PBLs were incubated with the indicated concentration of the β2AR antagonist ICI-118551 for 10 minutes, treated 30 minutes with the indicated concentration of ISO, and then stimulated 18 hours with PHA + CD28 mAb + IL-2. MFI values of CD25 expression (background subtracted) in gated CRTH2+ (□) and CRTH2 (▪) T cells are reported for 1 representative of 2 independent experiments.
As shown in Figure 2, a low level of PKA activation occurs within the context of CD3-stimulated cytokine production. To further explore the relevance of low levels of PKA activity on cytokine production, the concentration-dependent effects of PGE2, ISO, and db-cAMP on CD3-stimulated IL-13 production were assessed (Figure 5D). IL-13 production was inhibited by PGE2 similarly at concentrations of 100 nM and 1 μM, whereas 10 nM PGE2 did not have a significant effect. The lowest concentration of PGE2 tested (1 nM) enhanced CD3-stimulated IL-13 production in each experiment performed. For β-agonist, 100 nM to 10 μM ISO inhibited IL-13 production, whereas at 10 nM and 1 nM ISO, CD3-stimulated IL-13 production was enhanced in each experiment performed. For db-cAMP, a 5-nM concentration enhanced CD3-stimulated IL-13 production, suggesting that generation of relatively low levels of cAMP/PKA activity by PGE2 and β-agonist enhance IL-13 production. Conversely, low concentrations of PGE2, ISO, and db-cAMP did not increase CD3-stimulated IL-2 or IFN-γ production (not shown). PMA + calcimycin-stimulated IL-13 production, increased at saturating concentrations of PGE2 and db-cAMP, was not further increased by submaximal concentrations of these agents (not shown). Collectively, these data suggest that low levels of PKA activity are permissive for CD3-stimulated IL-13 production, whereas past a critical threshold of PKA activity IL-13 production is inhibited.
Modulation of MAPK and NF-κB signaling by PGE2 and β-agonist
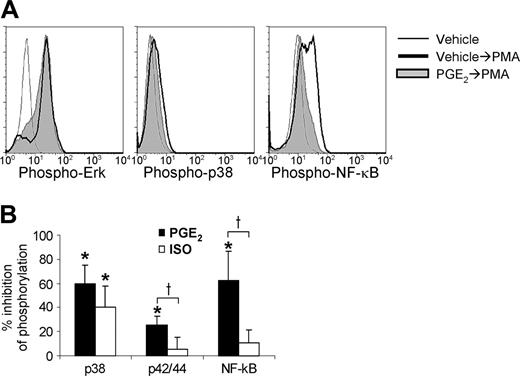
As noted in the previous section, PGE2 and ISO inhibited IFN-γ production, and PGE2 enhanced IL-13 production even when the upstream CD3-stimulated signaling events were bypassed by stimulation with PMA + calcimycin. In an attempt to identify signaling elements altered under these conditions, the effects of PGE2 and ISO on PMA-induced phosphorylation of the p38 MAPK, p42/44 MAPK (ERK), and NF-κB p65 were examined (Figure 6). Both PGE2 and ISO significantly inhibited PMA-stimulated phosphorylation of p38 MAPK (60% ± 15% and 41% ± 18%, respectively). PGE2 similarly inhibited NF-κB phosphorylation (63% ± 24%) but inhibited p42/44 phosphorylation to a lesser extent (25% ± 8%). Inhibition of p42/44 and NF-κB p65 by PGE2 was significantly greater than inhibition by ISO, which only minimally inhibited p42/44 and NF-κB p65 phosphorylation (6% ± 10% and 11% ± 11%, respectively).
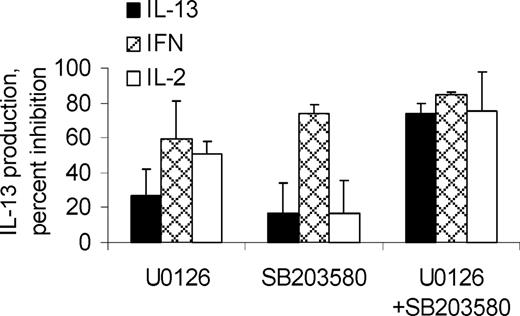
To clarify the roles of p38 and p42/44 in PMA-stimulated cytokine production, cells were pretreated with SB203580 (a selective p38 inhibitor51 ), U0126 (selective for mitogen-activated, extracellular-signal-regulated kinase-activating kinase [MEK], the upstream activator of p42/4452 ), or both (Figure 7). Inhibition of IL-13 production by either U0126 or SB203580 alone was minimal. However, the combination of both MAPK inhibitors decreased IL-13 production by 74% ± 6%. IFN-γ production was inhibited similarly by U0126 and SB203580, either alone (59% ± 22% and 74% ± 5%, respectively) or in combination (85% ± 3%). IL-2 production was inhibited by U0126 (51% ± 7%), less so by SB203580 (17% ± 19%), and the combination of inhibitors was only modestly more effective than was U0126 alone (75% ± 22%).
Collectively, these data indicate that IL-13 production requires either p38 or p42/44 activation, but not necessarily both; and IL-2 production is largely dependent on p42/44, whereas IFN-γ production requires activation of both p38 and p42/p44. Inhibition of p38 by either ISO or PGE2 appears sufficient to strongly inhibit PMA-stimulated IFN-γ production. The failure of PGE2 or ISO to inhibit IL-13 production can be explained by their inability to sufficiently inhibit p42/p44. Conversely, the strong inhibition of IL-2 production by PGE2 and ISO may be mediated in part by inhibition of p38 and p42/p44 but likely involves inhibition of other signaling elements.
Modulation of cytokine production by β-agonist and PGE2. (A,B) PBLs were cultured 14 days with IL-2 + PHA + CD28mAb ± IL-4 (IL-4 was not used in cultures for experiments testing IFN-γ production). After culture, the cells were treated 15 minutes with vehicle, PGE2 (1 μM), or ISO (10 μM), and then stimulated either 5 hours with PMA + calcimycin + IL-2 or 18 hours with CD3 + CD28 mAb + IL-2 (+monensin). Cytokine production was analyzed by flow cytometry (filled region, staining for IL-13 expression; open region, background from isotype control mAb—same for vehicle and PGE2 treated). To allow comparison of results among subjects (“Statistical analyses” section in “Materials and methods”), MFI of cytokine staining (background subtracted) was normalized as the percent change relative to MFI of the respective stimulus only (vehicle treated = 0-change). Histograms of cytokine production by T cells for a representative experiment for PGE2 are shown in panel A. (B) Reported are mean ± SD, n = 9 for IL-13 and IL-2, n = 6 for IFN-γ. *P < .05 for difference from 0 to change. (C) The experiments described in panel B were performed replacing PGE2/ISO with 200 μM db-cAMP (n = 5-7). (D) PBLs were treated 15 minutes with vehicle, PGE2, ISO, or db-cAMP at the indicated concentrations, then stimulated 18 hours with CD3 + CD28 + IL-2 for cytokine production. IL-13 production was analyzed, and MFI normalized to vehicle-treated cells as described in Figure 5B. Data represent mean ± SD from 6 independent experiments using PBLs freshly isolated (n = 3 for PGE2- and ISO treated; n = 2 for db-cAMP treated) or after culture for 14 days with IL-2 + IL-4 ± PHA + CD28 mAb (n = 3 for PGE2 and ISO treated; n = 1 for db-cAMP treated). Because effects were nearly identical for both populations, data were combined and fitted to a sigmoidal dose-response curve. Increased IL-13 production with 1 nM PGE2, 1 to 10 nM ISO, and 5 μM db-cAMP was observed in all 6 of the independent experiments performed. (E) PBLs were cultured 14 days with IL-2 + PHA + CD28 mAb + IL-4. After culture, the cells were treated 15 minutes with vehicle, PGE2, or ISO at the indicated concentrations, and then stimulated 18 hours with CD3 + CD28 mAb + IL-2 (without monensin). Culture supernatants were then analyzed by flow cytometry. The levels of IL-13 are reported as ng/mL (limit of detection: 20 pg/mL) for 1 representative of 2 independent experiments. (F) After culture of PBLs as in panel A, cells were incubated 5 minutes with ICI-188551 or CGP20712A, treated 15 minutes with subsaturating concentration of ISO (100 nM), then stimulated 18 hours with CD3 + CD28 mAb, IL-2, and monensin. After stimulation, IL-13 production was determined by flow cytometry. Reported is the net MFI for IL-13 production (MFI from isotype control staining subtracted) for 1 representative of 2 independent experiments. (G) PBLs were cultured as described in panels A and B, treated 15 minutes with vehicle or 100 nM ISO, then stimulated 18 hours with CD3 + CD28 mAb, IL-2, and monensin. IL-13 production and expression of CD3 and CD8 were determined by flow cytometry. MFI for IL-13 levels (isotype-control MFI subtracted) is reported for CD8+ and CD4+ (= CD8-) T cells for 1 representative of 3 independent experiments.
Modulation of cytokine production by β-agonist and PGE2. (A,B) PBLs were cultured 14 days with IL-2 + PHA + CD28mAb ± IL-4 (IL-4 was not used in cultures for experiments testing IFN-γ production). After culture, the cells were treated 15 minutes with vehicle, PGE2 (1 μM), or ISO (10 μM), and then stimulated either 5 hours with PMA + calcimycin + IL-2 or 18 hours with CD3 + CD28 mAb + IL-2 (+monensin). Cytokine production was analyzed by flow cytometry (filled region, staining for IL-13 expression; open region, background from isotype control mAb—same for vehicle and PGE2 treated). To allow comparison of results among subjects (“Statistical analyses” section in “Materials and methods”), MFI of cytokine staining (background subtracted) was normalized as the percent change relative to MFI of the respective stimulus only (vehicle treated = 0-change). Histograms of cytokine production by T cells for a representative experiment for PGE2 are shown in panel A. (B) Reported are mean ± SD, n = 9 for IL-13 and IL-2, n = 6 for IFN-γ. *P < .05 for difference from 0 to change. (C) The experiments described in panel B were performed replacing PGE2/ISO with 200 μM db-cAMP (n = 5-7). (D) PBLs were treated 15 minutes with vehicle, PGE2, ISO, or db-cAMP at the indicated concentrations, then stimulated 18 hours with CD3 + CD28 + IL-2 for cytokine production. IL-13 production was analyzed, and MFI normalized to vehicle-treated cells as described in Figure 5B. Data represent mean ± SD from 6 independent experiments using PBLs freshly isolated (n = 3 for PGE2- and ISO treated; n = 2 for db-cAMP treated) or after culture for 14 days with IL-2 + IL-4 ± PHA + CD28 mAb (n = 3 for PGE2 and ISO treated; n = 1 for db-cAMP treated). Because effects were nearly identical for both populations, data were combined and fitted to a sigmoidal dose-response curve. Increased IL-13 production with 1 nM PGE2, 1 to 10 nM ISO, and 5 μM db-cAMP was observed in all 6 of the independent experiments performed. (E) PBLs were cultured 14 days with IL-2 + PHA + CD28 mAb + IL-4. After culture, the cells were treated 15 minutes with vehicle, PGE2, or ISO at the indicated concentrations, and then stimulated 18 hours with CD3 + CD28 mAb + IL-2 (without monensin). Culture supernatants were then analyzed by flow cytometry. The levels of IL-13 are reported as ng/mL (limit of detection: 20 pg/mL) for 1 representative of 2 independent experiments. (F) After culture of PBLs as in panel A, cells were incubated 5 minutes with ICI-188551 or CGP20712A, treated 15 minutes with subsaturating concentration of ISO (100 nM), then stimulated 18 hours with CD3 + CD28 mAb, IL-2, and monensin. After stimulation, IL-13 production was determined by flow cytometry. Reported is the net MFI for IL-13 production (MFI from isotype control staining subtracted) for 1 representative of 2 independent experiments. (G) PBLs were cultured as described in panels A and B, treated 15 minutes with vehicle or 100 nM ISO, then stimulated 18 hours with CD3 + CD28 mAb, IL-2, and monensin. IL-13 production and expression of CD3 and CD8 were determined by flow cytometry. MFI for IL-13 levels (isotype-control MFI subtracted) is reported for CD8+ and CD4+ (= CD8-) T cells for 1 representative of 3 independent experiments.
Discussion
In this study we demonstrate that type 2 T cells directly respond to β-agonist to activate PKA and inhibit CD3-stimulated IL-13 production. Although both β-agonist and PGE2 signal through GS-coupled GPCRs to stimulate PKA activity, there were disparate effects of the 2 agents on T-cell cytokine production. The inhibition of CD3-stimulated IFN-γ, IL-2, and IL-13 production by PGE2 was greater than the inhibition by ISO in all cases. This lesser inhibitory effect of ISO was associated with a lower level of PKA activation. Interestingly, concentration-dependent responses for all agents tested suggested a biphasic effect of PKA activity on CD3-stimulated IL-13 production, and comparison of the regulation of CD3 versus PMA-stimulated IL-13 production suggests that PKA acts at both upstream and downstream signaling events that promote IL-13 but inhibit IFN-γ and IL-2 production.
The reports in the literature on the effects of β-agonists on T-cell cytokine production provide conflicting results. β-agonists have been reported to inhibit CD3-stimulated IFN-γ but not IL-4 expression in human PBLs, determined by reverse transcriptase-polymerase chain reaction (RT-PCR).18 Similar results were reported for antigen-stimulated murine T-cell clones, associated with detection of β2ARs on type 1 but not type 2 clones.17 However, others have shown that β-agonists inhibit CD3-stimulated IL-4, IL-5, and IFN-γ production in freshly isolated human PBLs but not in Th2- and Th1-polarized T-cell cultures.20 Goleva et al reported that a combination of β-agonist with low-dose steroids inhibits PHA-stimulated proliferation and IL-13 production by human PBMCs.19 However, the contribution of non-T cells to cytokine production could not be established because analyses were not performed at the single-cell level. The disparity among these studies may reflect the diversity of starting populations of T cells, effective concentrations of β-agonist used, and the measurement of functions at the population versus single-cell level.
Regulation of p42/p44, p38, and NF-κB phosphorylation in T cells. PHA-stimulated PBLs were cultured 14 days. After culture, the T cells were treated 15 minutes with vehicle, PGE2 (1 μM), or ISO (10 μM), then stimulated 10 minutes with 5 nM PMA. The cells were then analyzed for phosphorylation of ERK, p38, and NF-κB by flow cytometry. (A) Representative histograms are shown for the effects of PGE2 on PMA-induced phosphorylation. (B) The percent inhibition (mean ± SD, n = 4-6) of PMA-induced phosphorylation is presented for each condition (bottom). *P < .05 versus zero-inhibition; †P < .05 for effects of PGE2 versus ISO.
Regulation of p42/p44, p38, and NF-κB phosphorylation in T cells. PHA-stimulated PBLs were cultured 14 days. After culture, the T cells were treated 15 minutes with vehicle, PGE2 (1 μM), or ISO (10 μM), then stimulated 10 minutes with 5 nM PMA. The cells were then analyzed for phosphorylation of ERK, p38, and NF-κB by flow cytometry. (A) Representative histograms are shown for the effects of PGE2 on PMA-induced phosphorylation. (B) The percent inhibition (mean ± SD, n = 4-6) of PMA-induced phosphorylation is presented for each condition (bottom). *P < .05 versus zero-inhibition; †P < .05 for effects of PGE2 versus ISO.
Compared with Th1/Th2-polarized T-cell lines and clones, freshly isolated adult PBLs more closely represent the responding effector or memory T-cell populations in human adults because most adult human type 1 and type 2 T cells are effector or memory cells.53 T cells from short-term cultures of PBLs represent recently activated T cells. The advantage of testing the effects of agents ex vivo rather than in vivo is the ability to control the system and determine direct effects on T cells, at the single-cell level, rather than indirect effects modulated by other cell types responding to the agents. The approach employed in the current study takes into account all of these aspects by using freshly isolated human PBLs and short-term cultured T cells, a full range of β-agonist concentrations, and determination of T-cell cytokine production at the single-cell level. Furthermore, direct comparisons to the effects of PGE2, another Gs-coupled receptor agonist, and db-cAMP to specifically mimic elevated cAMP levels were made for β-agonist-mediated modulation of cytokine production and associated signaling events. The results strongly refute the notion that type 2 T cells do not directly respond to β-agonist.
It had been previously reported that human type 2 T cells,19,20 but not murine Th2 clones,17 express β2AR mRNA. However, functionally relevant levels of protein expression had not been established. The inability to detect β2ARs on type 2 T cells perhaps relates to a lack of sensitive tools/approaches for directly measuring expression or function of this low-abundance protein. In the present study, phosphorylation of VASP in CRTH2+ T cells on stimulation with β-agonist provides direct, unequivocal evidence that type 2 T cells express functionally relevant levels of β2AR capable of activating PKA. Antagonism of VASP phosphorylation by ICI-118551 demonstrated that the β2AR is the βAR subtype that mediates β-agonist-stimulated PKA activity in T cells. Most importantly, β-agonist directly inhibited CD3-stimulated IL-13 production by type 2 T cells, regardless of whether cells were freshly isolated, cultured short-term with IL-2 or PHA + CD28 mAb + IL-2 + IL-4, or CD2-/lo T cells that are mostly IL-13+ IFN-γ-.
β-agonist and PGE2 (via GS-coupled GPCRs) as well as CD3-mediated stimulation all were shown to promote PKA activation. Our results suggest that the regulation of T-cell functions by PKA is complex. In various cell types, PKA has been shown to not only attenuate mitogenic signaling by inhibition of several molecules, but also potentiate signaling by release of inhibitory molecules. Most studies have implicated CD3-independent activation of PKA activity by Gs-coupled GPCR-activating agonists, cAMP analogs, and cAMP-elevating agents (forskolin, phosphodiesterase inhibitors) as being inhibitory to TCR-mediated activation.54 The best-characterized mechanism of inhibition of CD3-mediated signaling specifically mediated by PKA is inhibition of activation of p56lck by Csk. Other mechanisms of regulation of signaling by PKA have been suggested, though the relevance to regulation of T-cell functions has not been well established. These mechanisms include inhibition of Raf-1 activity by PKA-mediated phosphorylation of Ser4355 and countering of calcineurin-mediated dephosphorylation of NF-AT by phosphorylation of NF-AT by PKA.56 In contrast, PKA may potentially promote transcription by releasing hematopoietic protein tyrosine phosphatase (HePTP) from p42/p44 and p38, allowing them to be more efficiently phosphorylated.57,58 There are conflicting reports on whether PKA enhances59,60 or inhibits42,61 the transactivating capacity of NF-κB p65.
We demonstrate here that CD3-stimulated IL-13 production, like IFN-γ and IL-2 production, is inhibited by ISO and PGE2, though more effectively by PGE2. Inhibition of IL-13 production is less efficient than inhibition of IFN-γ and IL-2 production by ISO or PGE2. The inhibition of production of each of these cytokines is likely mediated by the PKA-Csk mechanism. However, other mechanisms also may contribute to further inhibit IFN-γ and IL-2 production. ISO and PGE2 inhibited PMA + calcimycin-stimulated IFN-γ and IL-2 production, suggesting that modulation of signals that can occur downstream of the p56lck-Csk locus could further inhibit IFN-γ and IL-2 production. Such a mechanism is not expected for IL-13 because PGE2 enhances PMA + calcimycin-stimulated IL-13 production. Rather, modulation of downstream signaling by PGE2 could potentially offset the PKA-Csk block in upstream signaling for IL-13 production.
MAPK dependence for cytokine production. IL-2-cultured PBLs were treated 15 minutes with vehicle, U0126 (10 μM), or SB203580 (3 μM), then stimulated 5 hours with PMA + calcimycin + IL-2 + monensin. Indicated is the percent inhibition of cytokine production by T cells, relative to cells treated only with vehicle before stimulation (n = 3).
MAPK dependence for cytokine production. IL-2-cultured PBLs were treated 15 minutes with vehicle, U0126 (10 μM), or SB203580 (3 μM), then stimulated 5 hours with PMA + calcimycin + IL-2 + monensin. Indicated is the percent inhibition of cytokine production by T cells, relative to cells treated only with vehicle before stimulation (n = 3).
PMA-stimulated IL-13 production is not inhibited by ISO and is actually enhanced by PGE2, even though IFN-γ and IL-2 production is inhibited. The inhibition of p38 activation by ISO or PGE2 was not expected to inhibit IL-13 production because inhibition of both p38 and p42/p44 activity was shown to be required to significantly inhibit IL-13 production. Based on our understanding of transcriptional regulation of IL-4,62,63 one potential mechanism for the enhanced IL-13 production by PGE2 is increased phosphorylation of CREB by PKA to enhance transactivation with CREB-binding protein (CBP)/p300. Our preliminary studies suggest that PGE2, but not β-agonist, promotes significantly higher phospho-CREB levels than does PMA.
The inhibition of CD3-stimulated IL-13 production by β-agonist and PGE2 is not only lost at low concentrations of agonist, but also IL-13 production is actually increased. At these low concentrations of agonist, activation of PKA induced by PGE2 and ISO appears insufficient to induce Csk-dependent inhibition of p56lck. In the absence of inhibition by Csk, the downstream signaling events that promote IL-13 production may be affected in a manner similar to how PGE2 affects PMA-stimulated IL-13 production, possibly by PKA-mediated CREB phosphorylation. Consistent with our findings, Ozegbe et al recently reported a trend that low-dose PGE2 enhances antigen-stimulated IL-5 production by mouse lymph node T cells.64 In vivo, T cells are likely exposed to a wide range of levels of PGE2 in a paracrine manner, depending on the intensity of local inflammation and the complement of infiltrating inflammatory cells.
The inhibition of PMA-stimulated IFN-γ production by PGE2 and β-agonist can readily be accounted for by inhibition of p38 phosphorylation, originally described here, because IFN-γ production is dependent on p38 activation, as described here and by others (reviewed in Rincon65 ). Inhibition of NF-κB and p42/p44 activation likely is not involved because ISO minimally affected their activation. Such a mechanism is less certain for the inhibition of IL-2 production because PMA-stimulated IL-2 production was much less sensitive to inhibition of p38 activity. Inhibition of NF-κB and p42/p44 activation is probably not involved for the same reasons described for inhibition of IFN-γ production. A potential mechanism for inhibition of IL-2 production is high levels of phospho-CREB. In anergic T cells (defined as being defective in proliferative responses), levels of activated CREB are elevated, leading to transcriptional inhibition and defective IL-2 production.66 The PKA dependence on the inhibition of p38 phosphorylation and IFN-γ and IL-2 production is currently under investigation.
We show that T cells have a basal level of PKA activity, and CD3- and PMA-mediated stimulation increases PKA activity by approximately 2- and 3-fold, respectively, dependent on PKC. An association of elevated PKA activity and TCR-mediated activation with co-stimulation with IL-1β, dependent on PKC, had been previously reported.41,67 However, the role of basal or CD3-stimulated PKA activity in T-cell activation for cytokine production remains controversial. Several groups suggest that basal or CD3-stimulated PKA activity is not required or may attenuate cytokine production,1,4 but others suggest that some level of PKA activity is required for efficient T-cell activation.68,69 We demonstrate for the first time that VASP is phosphorylated upon CD3-mediated stimulation, dependent on induced PKA activity.
Our findings underscore the importance of considering the complex effects of PKA-activating agonists used not only under experimental conditions, but also for therapeutics, as with β-agonist therapy for control of asthma. From the results in this study it is obvious that opposing results could be obtained for modulation of IL-13 production by PKA-activating agents, depending on the concentration of agent used and the stimulus used to elicit cytokine production. In terms of understanding the effects of endogenous or exogenously provided β-agonist or PGE2 on disease, the effective concentrations of the agents in the environment of the responding cells need to be considered to predict the outcome on the cells. At high local concentrations, as can occur with inhaled beta-agonist therapy,70,71 β-agonist could inhibit IL-13, IFN-γ, and IL-2 production by T cells responding to allergen, which could be beneficial for the asthmatic. However, if the concentration is below the threshold for inhibition, IL-13 production by T cells responding to allergen (or other antigens, such as viral or bacterial) could be enhanced—a potentially detrimental outcome for the asthmatic. Establishing the prevalence of these concentration-dependent effects by PKA-activating agonists in other inflammatory cell types will be important in understanding how such agonists impact the inflammatory milieu in vivo.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2005-08-3265.
Supported by National Institutes of Health grant HL58506.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal