The tumor milieu can influence dendritic cell (DC) differentiation. We analyzed DC differentiation in a 3-dimensional tumor model and propose a new mechanism of DC modulation by the tumor environment. Monocytes were cultured in the presence of IL-4 and GM-CSF within multicellular tumor spheroids (MCTSs) generated from different tumor cell lines. Monocytes invaded the MCTSs and differentiated into tumor-associated dendritic cells (TADCs). The antigen expression was altered on TADCs independent of the culture conditions (immature/mature DCs, Langerhans cells) and IL-12 secretion was reduced. Supernatants of MCTSs could partially transfer the suppressive effect. Conditioned media from urothelial carcinoma cell lines contained high levels of M-CSF and IL-6, both cytokines known to modulate DC differentiation. In contrast, melanoma and prostate carcinoma MCTS cocultures produced little M-CSF and IL-6, but high levels of lactic acid. Indeed, addition of lactic acid during DC differentiation in vitro induced a phenotype comparable with TADCs generated within melanoma and prostate carcinoma MCTSs. Blocking of lactic acid production in melanoma MCTS cocultures reverted the TADC phenotype to normal. We therefore conclude that tumor-derived lactic acid is an important factor modulating the DC phenotype in the tumor environment, which may critically contribute to tumor escape mechanisms.
Introduction
Tumor cells use several mechanisms to evade elimination by the immune system. Dendritic cells (DCs), which are important for the initiation of a specific antitumor T-cell response, are also possible target cells susceptible to tumor-mediated immunosuppression. Both circulating and tumor-infiltrating DCs from cancer patients appear to be phenotypically and functionally defective.1-6 Characterization of DCs in tumor sections gave ambiguous results. Tumor-associated dendritic cells (TADCs) in renal cell carcinoma were described as mature DCs7 ; for breast carcinoma, intratumoral TADCs have been found to be immature, while peritumoral TADCs showed a mature phenotype.8 The presence of high numbers of infiltrating CD1a+ cells seems to be associated with an improved prognosis in breast carcinoma.9,10
Immunosuppressive factors such as IL-10 have been demonstrated to influence the differentiation and maturation of DCs in vitro.11,12 Experiments with tumor supernatants revealed that IL-6 and M-CSF produced by tumor cells are important factors that inhibit DC differentiation.13 High levels of IL-4 could partially revert this effect.14 In animal models, VEGF inhibited the function of Langerhans cells,15 and the differentiation of human CD34+ cells from cord blood was also inhibited by VEGF.1 Hyperactivation of STAT3 seems to be involved in the inhibition of DC differentiation by tumor-derived cytokines.2
Most of these investigations used either tumor cell monolayers or supernatants of tumor cell monolayers, which only partially mimic the tumor microenvironment. Multicellular spheroids (MCTSs) represent a more complex and more in-vivo-like 3-dimensional tissue culture model for avascular sites of a tumor or of micrometastasis. It better mimics the in vivo situation found in a tumor than monolayer cultures with respect to growth kinetics, extracellular matrix, nutrient gradients, oxygen tension, and pH.16,17 Tumor cells found in the inner core of solid tumors are compromised in their accessibility to oxygen and rely more on glycolysis for ATP synthesis and survival. Hypoxia up-regulates genes encoding VEGF, glucose transporters, and glycolytic enzymes such as lactate dehydrogenase.18,19 Accordingly, those cells produce and secrete lactic acid, which in turn lowers the pH in the tumor environment. Most likely hypoxia-inducible factors (HIF-1a, HIF-2a), transcription factors that are induced either under hypoxic conditions or by cell transformation, are involved in this regulatory process.20,21 HIF-1a and HIF-2a are expressed in the majority of human tumors.22
Hypoxia has been shown to reduce CD80 on human monocytes,23 and recently Burke et al demonstrated that hypoxia alters the gene expression in monocyte-derived macrophages.24 In addition, the production of cytokines by DCs is influenced by oxygen tension.25
In this study we used the MCTS experimental model system to investigate the differentiation of human monocytes into DCs in the presence of IL-4 and GM-CSF. We found that hypoxia or pH reduction alone had little effect on DC differentiation in vitro. However, lactic acid altered antigen phenotype and functional activity of DCs and, alone or in combination with the tumor-derived cytokines M-CSF and IL-6, generated a special tumor-associated DC phenotype.
Materials and methods
Isolation and culture of monocytes
Monocytes were obtained by leukapheresis of healthy donors, followed by density gradient centrifugation over Ficoll/Hypaque and separation by countercurrent centrifugation (J6M-E centrifuge; Beckmann, Munich, Germany) as described previously.26,27 Approval for this article was obtained from the Department of Hematology and Oncology (University of Regensburg, Germany) institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Monocyte purity was at least 85% as determined by expression of the monocyte-specific antigen CD14. Isolated monocytes were either cultured alone for the differentiation of control DCs or cocultured with multicellular spheroids to obtain tumor-associated DCs. To exclude effects by donor variations, all experiments were performed with monocytes from at least 3 different healthy donors. Control DCs were generated by culturing monocytes at a density of 0.5 to 1 × 106 cells/mL in RPMI-1640 (Biochrom, Berlin, Germany) supplemented with antibiotics (50 U/mL penicillin and 50 mg/mL streptomycin), l-glutamine (2 mM; Gibco, Carlsbad, CA), 10% fetal calf serum (FCS; BioWhittaker, Walkersville, MD), 50 U/mL IL-4 (Promocell, Heidelberg, Germany), and 200 U/mL GM-CSF (Leukine/Sargramostim; Immunex, Seattle, WA). In order to induce differentiation of monocytes into Langerhans cells, we added 5 ng/mL transforming growth factor beta (TGF beta 1; PeproTech, Rocky Hill, NJ) in some experiments. To induce maturation of DCs, cells were cultured for 72 hours with 10 ng/mL LPS.
Generation of multicellular spheroids and spheroid cocultures
The cell lines J82 (urothelial carcinoma; ATCC, Manassas, VA),28 UMUC3 (urothelial carcinoma; ATCC), MelIm (melanoma), Mel108 (melanoma), and PC3 (prostate carcinoma) were grown in RPMI-1640 medium (Gibco) supplemented with 10% FCS (BioWhittaker) and additives as described in “Isolation and culture of monocytes.” Cells were cultured under standard tissue-culture conditions and regularly tested for Mycoplasma contamination (Mycoplasma detection kit; Biochrom).
Multicellular spheroids (MCTSs) were generated using the liquid overlay culture technique.29 In brief, 5 × 103 suspended cells from exponentially growing tumor cell monolayers were cultured on 1% solid agarose in 96-well plates. After 3 days of cultivation cells had formed tight aggregates. On day 5, half of the medium was replaced by a monocyte suspension containing 4 × 104 monocytes in medium supplemented with 10% FCS, 50 U/mL IL-4 (Promocell), and 200 U/mL GM-CSF (Leukine/Sargramostim; Immunex) per well. After 5 to 7 days of coculture, single-cell suspensions were prepared by incubation with 0.05/0.02% trypsin/EDTA (PAN, Aidenbach, Germany) for 5 minutes at 37°C.
To investigate the impact of tumor cell lactic acid production on DC differentiation in the coculture, MCTSs (J82 and MelIm) were generated in the presence or absence of oxamic acid (Sigma, Deisenhofen, Germany), an inhibitor of lactate dehydrogenase. To exclude the possibility that oxamic acid influences DCs directly, MCTSs were washed before the monocyte suspension was added. In these experiments MCTS cocultures were separated by vigorous pipetting as we wanted to determine CD14 expression (CD14 is eliminated by trypsin incubation).
Immunohistochemistry
Cocultures of MCTSs and monocytes were harvested from the agarose-coated wells, fixed in 4% formalin, paraffin embedded using a Hypercenter XP (Shandon, Pittsburgh, PA), and serially sectioned (5-6 μm). Paraffin sections were processed according to an established immunohistochemical protocol using the NexEs/HC module (Ventana Medical Systems, Tucson, AZ), monoclonal anti-human CD45 IgG (working concentration: 0.75 μg/mL; DAKOCytomation, Hamburg, Germany) and the iVIEW DAB (diaminobenzidine) detection kit (Ventana Medical Systems). All sections were counterstained with hematoxylin. Median spheroid sections were documented using an image processing system consisting of an inverted microscope (AxioVert 200) equipped with a digital camera (AxioCam MRc) and software (KS300) (all from Carl Zeiss, Göttingen, Germany). Images were taken with a magnification of 50 × and 200 × (objectives, 5 ×/0.12 numeric aperture [NA] and 20 ×/0.3 NA, respectively) and were further processed (cut out and white-balanced) using Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA). Respective scale bars are shown for each image.
Determination of antigen expression by flow cytometry
For determination of surface antigen expression, cell suspensions from cocultures were harvested at days 4 or 7, washed twice with cold phosphate-buffered saline (PBS; Gibco) containing 0.1% sodium azide and 0.6 mg/mL immunoglobulin, and immunostained with fluorescein-isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) anti-CD1a (clone T6-RD1), anti-HLA-DR (clone B-F1; Diaclone/Labonova, Besançon, France), anti-HLA-DQ (clone TÜ 169; BD Pharmingen, San Diego, CA), anti-CD86 (clone 2331; BD Pharmingen), anti-CD83 (clone HB15a; Immunotech, Marseille, France), anti-CD207 (clone DCGM4; Immunotech), anti-CD14 (My4; Beckman Coulter, Fullerton, CA), and IgG or IgM, as an isotype control (Beckman Coulter). Counterstaining was performed with a PerCP-conjugated anti-CD45 antibody (clone 2D1; BD Biosciences, San Jose, CA) to label all monocytic cells. After 2 wash steps, cells were fixed in 1% paraformaldehyde/PBS and analyzed by flow cytometry using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Fluorescence intensities of monocytic cells were analyzed by gating for CD45+ cells. Antigen expression is calculated by subtracting the median of the isotype control from the median of the specific staining. Statistical analysis was performed with the unpaired t test.
Determination of cytokines and lactate in tumor cell supernatants
Multicellular spheroids of the cell lines J82 and MelIm were grown in liquid overlay in RPMI-1640 medium (Gibco) supplemented with 10% FCS (BioWhittaker) and additives as described in “Isolation and culture of monocytes.” After 3 to 4 days of culture, half of the medium was replaced by either fresh medium or a monocyte suspension containing 4 × 104 monocytes in fresh medium supplemented with 10% FCS, 50 U/mL IL-4 (Promocell), and 200 U/mL GM-CSF (Leukine/Sargramostim; Immunex). Cumulative supernatants were harvested on day 7, filtered, and stored at -20°C. IL-6 and M-CSF were determined in the culture supernatants with commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Lactate in tumor cell supernatants was measured with a routine procedure using an ADVIA 1650 (Bayer, Tarrytown, NY) and specific reagents (Roche) (Department of Clinical Chemistry, University of Regensburg).
To demonstrate the impact of an inhibitor of lactate dehydrogenase on tumor cell lactic acid production, supernatants of monolayer cultures were generated in the presence or absence of oxamic acid (Sigma). Cells (5 × 104/2 mL) were grown in 6-well microtiter plates in RPMI-1640 medium with or without 70 mM oxamic acid and harvested after 4 to 6 days. Supernatants were stored at -20°C. Lactate in the tumor cell supernatants was determined with a routine procedure as described.
To analyze the IL-10 and IL-12 production of MelIm-TADCs or PC3-TADCs, we stimulated the MCTS cocultures for 72 hours with 10 ng/mL LPS. Supernatants were harvested and stored at -20 °C. IL-10 and IL-12 (p70) were determined with commercially available ELISA kits according to the manufacturer's instructions. Statistical analysis was performed with the Mann-Whitney test.
Dendritic cell differentiation in the presence of tumor cell supernatants, lactic acid, or hypoxia
Supernatants of MCTS cultures of J82 and MelIm or monolayer cultures were harvested and filtered before adding them to monocytes in the presence of IL-4 and GM-CSF. After 5 to 7 days of incubation, cells were harvested, dissociated, and immunostained for determination of antigen expression.
In order to evaluate the impact of lactic acid on the dendritic cell differentiation, l-lactic acid (Sigma) was added to the cultures at various concentrations. Retitration of the pH to physiologic 7.4 in the culture medium was performed with NaOH. For experiments mimicking hypoxic conditions, cells were incubated using 2% O2, 5% CO2, 37°C in a humidified atmosphere.
Dendritic cell activation in the presence of lactic acid
To demonstrate the influence of lactic acid on DC activation, DCs were generated as described in “Isolation and culture of monocytes” in RPMI 1640 (Biochrom) supplemented with GM-CSF and IL-4. DCs were harvested on day 7 of culture and 1 × 106 cells/2 mL were cultured with or without 100 ng/mL LPS in the absence or presence of various concentrations of l-lactic acid (Sigma). After 24 hours, supernatants were harvested, filtered, and stored at -20°C. IL-10 and IL-12 (p70) were determined in the culture supernatants with commercially available ELISA kits.
Generation and analysis of antigen-specific cytotoxic T lymphocyte (CTL) lines from PBMCs
Antigen-specific CTL lines were generated as described previously.30 Briefly, purified CD8+ T cells were stimulated with DCs pulsed with the appropriate peptide (30 μg/mL) and human β2-microglobulin (β2m; 10 μg/mL) in 96-well plates in complete medium supplemented with 10% human AB serum and 2% T-cell growth factor. TCGF was produced by stimulating peripheral blood mononuclear cells (PBMCs) with phytohemagglutinin (PHA), phorbol myristate acetate (PMA), and irradiated Epstein-Barr virus (EBV)-transformed B cells. After 2 hours, cells were washed and incubated for 40 hours, and supernatants were harvested and stored. Two percent TCGF contains 10 to 20 IU IL-2/mL.
After 3 cycles of stimulation, MHC multimer analysis revealed a frequency of 80% to 90% antigen-specific CTLs in the T-cell lines.
T cells were labeled with 2 μM carboxyfluorescein diacetate-succinimidyl ester (CFSE; Sigma) prior to restimulation with peptide-pulsed DCs in the absence or presence of lactic acid (10 mM, 20 mM). After 1 to 6 days of incubation, T cells were stained with anti-CD3-PerCP (clone SK7; BD Biosciences) and anti-CD25-APC (clone 2A3; BD Biosciences) mAbs and analyzed by flow cytometry for CFSE and CD25 expression of CD3 gated T cells.
Results
Phenotypical characterization of tumor-associated dendritic cells
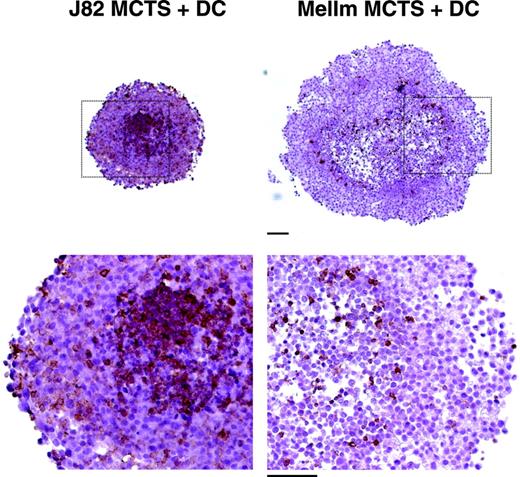
In previous experiments, we established an in vitro model for the generation of tumor-associated macrophages. Human monocytes cocultured with 3-dimensional MCTSs were found to invade MCTSs and showed a modulated antigen expression.31 To investigate the differentiation of TADCs in this tumor model, cocultures were performed in the presence of IL-4 and GM-CSF, both cytokines known to induce DC differentiation in vitro. For the generation of MCTSs, the 2 urothelial carcinoma cell lines J82 and UMUC3 and the melanoma cell lines Mel108 and MelIm were used. After 24 hours, monocytes had infiltrated the MCTS and were detected as CD45+ cells throughout the MCTS coculture. One representative experiment with MelIm-MCTS and J82-MCTS is shown in Figure 1. MelIm MCTSs were larger than the other MCTS types but the infiltration of monocytes was always stronger into the urothelial carcinoma MCTSs than into melanoma MCTSs.
To determine the phenotype of the invaded cells, antigen expression of CD45+ cells in MCTS-monocyte cocultures was examined by flow cytometry in comparison with control DCs generated without tumor contact. MCTS cocultures were separated with trypsin/EDTA, and TADCs were labeled with mouse anti-human CD45 antibody in combination with antibodies against CD1a, HLA-DR, HLA-DQ, CD80, CD83, CD86, and CD16. Trypsination of control DCs had no effect on the expression of these antigens but eliminated CD14 expression (data not shown). For flow cytometry, CD45+ cells were gated. In comparison with control DCs, CD1a expression was significantly reduced (unpaired t test, control DCs vs J82 TADCs [P < .001], control DCs vs UMUC3 TADCs/Mel108 TADCs/MelIm TADCs [P < .05]) on all types of TADCs generated within urothelial and melanoma MCTSs (Figure 2A). We also found a similar reduction of CD1a within prostate carcinoma MCTSs (PC3 cell line, data not shown). HLA-DR expression showed a heterogeneous regulation depending on the MCTS type, and a significant (down)regulation was found only for J82 MCTS (Figure 2A). In contrast, CD80, CD86, and CD16 were up-regulated by tumor cell contact and no effect was detected for CD83 expression (data not shown). CD14, which is normally down-regulated during the differentiation of monocytes into DCs, was still expressed on TADCs as determined by immunohistochemistry (data not shown).
Infiltration of monocytes into multicellular spheroids of J82 and MelIm. MCTSs were generated using 5 × 103 suspended cells from exponentially growing tumor cell monolayers and cultured on 1% solid agarose in 96-well plates. After 4 to 5 days, half of the medium was replaced by a monocyte suspension containing 4 × 104 monocytes. MCTS cocultures were harvested 1 day later. Images show 5-μm paraffin sections stained for the leukocyte marker CD45 using a routine peroxidase technique with DAB as chromogen and hematoxylin counterstain to visualize infiltrated monocytes (bar = 100 μm).
Infiltration of monocytes into multicellular spheroids of J82 and MelIm. MCTSs were generated using 5 × 103 suspended cells from exponentially growing tumor cell monolayers and cultured on 1% solid agarose in 96-well plates. After 4 to 5 days, half of the medium was replaced by a monocyte suspension containing 4 × 104 monocytes. MCTS cocultures were harvested 1 day later. Images show 5-μm paraffin sections stained for the leukocyte marker CD45 using a routine peroxidase technique with DAB as chromogen and hematoxylin counterstain to visualize infiltrated monocytes (bar = 100 μm).
TGF beta 1 is known to induce a particular type of antigen-presenting cell (ie, Langerhans cells). To analyze whether the inhibitory effect of MCTSs is also found under these experimental conditions, MelIm MCTSs were cultured with monocytes in the presence of IL-4, GM-CSF, and TGF beta 1. TGF beta 1 did not revert the inhibitory effect of the tumor environment. In addition, we determined whether maturation of DCs with LPS for 72 hours could rescue the phenotype. Indeed, CD1a expression was still suppressed and CD83 and HLA-DR were not up-regulated by LPS in TADCs in MCTS cocultures compared with control DCs without tumor contact (Figure 2B). These results show that the inhibition of the DC phenotype in the MCTSs is found under various DC culture conditions.
Antigen expression of TADCs cultured under different conditions. The urothelial carcinoma cell lines J82 and UMUC3 and the melanoma cell lines MelIm and Mel108 were used for MCTS generation. After 4 to 5 days, half of the medium was replaced by a monocyte suspension in IL-4 and GM-CSF (A) or in IL-4, GM-CSF, and TGF beta 1 (B). In some experiments, LPS was added during the last 72 hours to induce DC maturation (B). After 5 to 7 days in coculture, MCTSs were dissociated and stained with mouse anti-human CD45 antibody in combination with antibodies against the indicated antigens. The same staining procedure was performed with control DCs. For flow cytometry analysis, CD45+ cells were gated. Panel A shows the mean and SEM of the median fluorescence intensity (isotype subtracted) of at least 3 experiments (CD1a expression: control DCs versus J82 TADCs [P < .001], control DCs vs UMUC3 TADCs/Mel108 TADCs/MelIm TADCs [P < .05], unpaired t test). Panel B represents 1 representative experiment of 3. Quadrant statistics are shown for the top right quadrant (CD45+ cells were gated and represent 100%).
Antigen expression of TADCs cultured under different conditions. The urothelial carcinoma cell lines J82 and UMUC3 and the melanoma cell lines MelIm and Mel108 were used for MCTS generation. After 4 to 5 days, half of the medium was replaced by a monocyte suspension in IL-4 and GM-CSF (A) or in IL-4, GM-CSF, and TGF beta 1 (B). In some experiments, LPS was added during the last 72 hours to induce DC maturation (B). After 5 to 7 days in coculture, MCTSs were dissociated and stained with mouse anti-human CD45 antibody in combination with antibodies against the indicated antigens. The same staining procedure was performed with control DCs. For flow cytometry analysis, CD45+ cells were gated. Panel A shows the mean and SEM of the median fluorescence intensity (isotype subtracted) of at least 3 experiments (CD1a expression: control DCs versus J82 TADCs [P < .001], control DCs vs UMUC3 TADCs/Mel108 TADCs/MelIm TADCs [P < .05], unpaired t test). Panel B represents 1 representative experiment of 3. Quadrant statistics are shown for the top right quadrant (CD45+ cells were gated and represent 100%).
Analysis of potential mechanisms responsible for the modulation of DC antigen expression in the tumor microenvironment
It is known that cytokines influence the phenotype of DCs. Therefore, we first determined which cytokines were secreted by tumor cells in MCTS culture and cytokine levels in MCTS/monocyte cocultures. Tumor cells cultured as MCTSs did not secrete IL-10, TGF beta 1, TNF-alpha, IFN-gamma, IL-12, or IL-1 beta (data not shown). However, as recorded in Table 1, J82-MCTS culture supernatants contained high amounts of M-CSF and IL-6. In contrast, MelIm MCTSs secreted very low amounts of M-CSF and IL-6. Here, a significant secretion of M-CSF and IL-6 could be detected only when MelIm MCTSs were cocultured with monocytes. The same was true for the prostate carcinoma cell line PC3 (37 ± 5 pg/mL for IL-6, 123 ± 50 pg/mL for M-CSF in the MCTS coculture). This suggests that monocytes themselves produce these cytokines in the MCTS cocultures. Accordingly, also DC control flasks contained significant amounts of M-CSF (data not shown), which is most likely important for monocyte survival. Due to the endogenous M-CSF production in DCs, it is impossible to establish MCTS cocultures free of M-CSF and IL-6.
Soluble factors secreted by MCTSs with or without monocyte coculture
. | M-CSF, pg/mL* . | IL-6, pg/mL . | Lactate, mg/dL . |
|---|---|---|---|
| MelIm MCTSs† | 34 ± 3 | < 3 | 98 ± 12 (10.8 mM) |
| MelIm MCTSs and monocytes | 171 ± 40 | 23 ± 4 | ND |
| J82 MCTSs | 1367 ± 289 | 12 150 ± 488 | 18 ± 2 (2 mM) |
| J82 MCTSs and monocytes | 583 ± 167 | 9 241 ± 1894 | ND |
. | M-CSF, pg/mL* . | IL-6, pg/mL . | Lactate, mg/dL . |
|---|---|---|---|
| MelIm MCTSs† | 34 ± 3 | < 3 | 98 ± 12 (10.8 mM) |
| MelIm MCTSs and monocytes | 171 ± 40 | 23 ± 4 | ND |
| J82 MCTSs | 1367 ± 289 | 12 150 ± 488 | 18 ± 2 (2 mM) |
| J82 MCTSs and monocytes | 583 ± 167 | 9 241 ± 1894 | ND |
Data represent the mean ± SEM of at least 3 independent experiments with different monocyte donors.
ND indicates not determined.
IL-6 and M-CSF were determined by ELISA. Lactate was measured with a clinical routine procedure
MCTSs were cultured with or without monocytes for 7 days. Supernatants of 20 wells were pooled, filtered, and stored at –20°C
A striking difference between J82 and MelIm MCTS culture systems was the pH of the medium at the end of the culture (day 7). Differences in the pH could be explained by both a higher proliferation rate and an enhanced aerobic glycolysis in MelIm cultures. Aerobic glycolysis is accompanied by an up-regulation of lactate dehydrogenase and production of lactic acid. We therefore analyzed the lactate levels in MCTS supernatants and found that the lactate content was about 5 times higher in MelIm MCTS supernatants (Table 1). Such high lactate levels were also found in PC3 MCTS cultures (data not shown).
Hypoxia is one possible regulator of lactate dehydrogenase and normally induces the production of lactic acid. Accordingly, the hypothesis that DCs may show an altered phenotype under hypoxic conditions had to be proved. However, when comparing DCs cultured in 2% O2 with normoxic conditions (20% O2), only marginal effects were found regarding antigen expression and functional activity (data not shown). This indicates that moderate hypoxia per se does not modulate DC differentiation but may act indirectly through the modulation of the tumor cell.
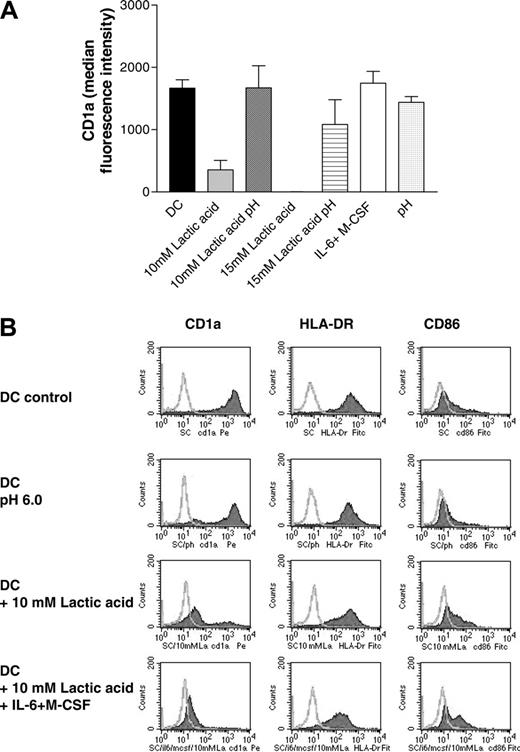
Next we differentiated DCs in the presence or absence of M-CSF (1 ng/mL), IL-6 (10 U/mL), and lactic acid (10 mM, 15 mM) corresponding to concentration ranges measured in the MCTS supernatants. Figure 3A demonstrates that IL-6 and M-CSF do not modulate CD1a expression at these low concentrations, whereas 10 mM lactic acid associated with a decrease in the pH clearly inhibited DC differentiation (Figure 3A-B). Changes of the pH alone during the DC differentiation process had only a marginal effect on the antigen expression profile of DCs (Figure 3A-B). A combination of all 3 factors—lactic acid, IL-6, and M-CSF— further decreased CD1a expression in some experiments (Figure 3B). We conclude that effective concentrations of IL-6 and M-CSF are generated only within the J82-MCTS coculture system with direct cell-cell and cell-matrix interactions.
Effect of lactic acid and low doses of M-CSF and IL-6 on CD1a expression (A). Monocytes were differentiated in the presence of IL-4 and GM-CSF for 7 days with or without the addition of 10 to 15 mM lactic acid or low-dose M-CSF (1 ng/mL) and IL-6 (10 U/mL). As a control, the medium with IL-4 and GM-CSF was titrated to pH 6.0 to 6.5 with HCl (pH). In some samples, the pH of the lactic acid-containing cultures (10 mM lactic acid pH 6.5, 15 mM lactic pH 6.3) was adjusted to pH 7.4. Cells were stained with an antibody against CD1a and analyzed with a FACScan. The figure shows the mean and SEM of the median fluorescence intensity (isotype subtracted) of at least 3 experiments. (B) Phenotypical characterization of DCs cultured under different conditions. Monocytes were differentiated in the presence of IL-4 and GM-CSF for 7 days with or without the addition of 10 mM lactic acid, low-dose M-CSF (1 ng/mL), and IL-6 (10 U/mL), or a combination of lactic acid, low-dose M-CSF, and IL-6. As a control, monocytes were cultured in a medium with pH 6.0. Cells were stained with an antibody against CD1a, HLA-DR, and CD86 and analyzed with a FACScan. Filled histograms represent the specific staining.
Effect of lactic acid and low doses of M-CSF and IL-6 on CD1a expression (A). Monocytes were differentiated in the presence of IL-4 and GM-CSF for 7 days with or without the addition of 10 to 15 mM lactic acid or low-dose M-CSF (1 ng/mL) and IL-6 (10 U/mL). As a control, the medium with IL-4 and GM-CSF was titrated to pH 6.0 to 6.5 with HCl (pH). In some samples, the pH of the lactic acid-containing cultures (10 mM lactic acid pH 6.5, 15 mM lactic pH 6.3) was adjusted to pH 7.4. Cells were stained with an antibody against CD1a and analyzed with a FACScan. The figure shows the mean and SEM of the median fluorescence intensity (isotype subtracted) of at least 3 experiments. (B) Phenotypical characterization of DCs cultured under different conditions. Monocytes were differentiated in the presence of IL-4 and GM-CSF for 7 days with or without the addition of 10 mM lactic acid, low-dose M-CSF (1 ng/mL), and IL-6 (10 U/mL), or a combination of lactic acid, low-dose M-CSF, and IL-6. As a control, monocytes were cultured in a medium with pH 6.0. Cells were stained with an antibody against CD1a, HLA-DR, and CD86 and analyzed with a FACScan. Filled histograms represent the specific staining.
Lactate is transported into the cell by the monocarboxylate transporter MCT-1 and this process is strictly pH dependent.32 Therefore, we analyzed whether adjusting the pH would alter the effect of lactic acid. As shown in Figure 3A, the inhibitory effect of 10 mM lactic acid could indeed be reverted by adjusting the pH to 7.4, indicating that the effect of lactate depends on its transport into the cell. At concentrations higher than 10 mM we could only partially counteract the effect of lactic acid by adjusting the pH, which implies that other mechanisms are involved at higher concentrations that act independently of the MCT-1 transport system.
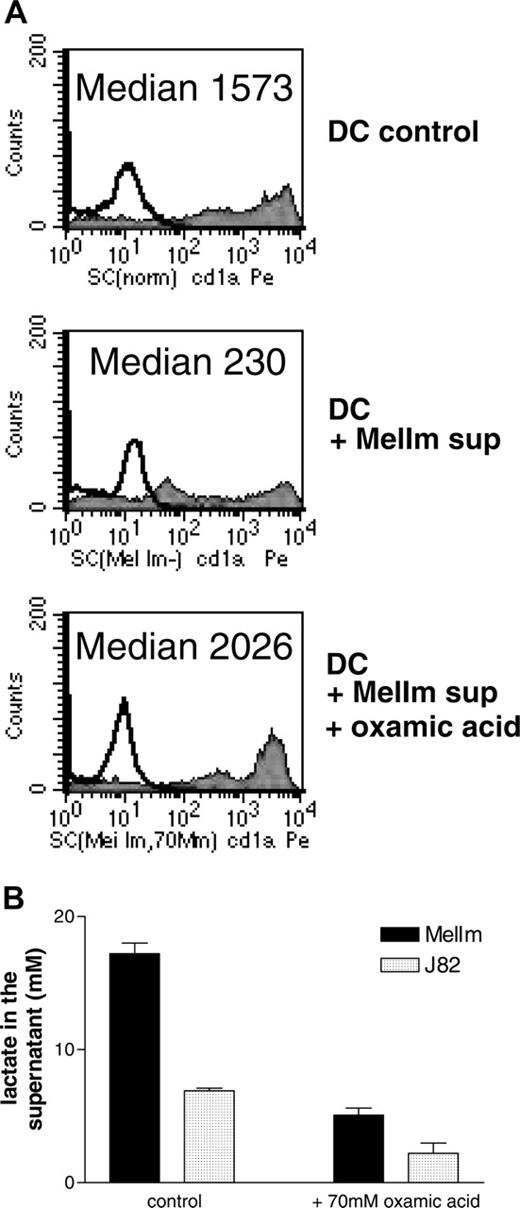
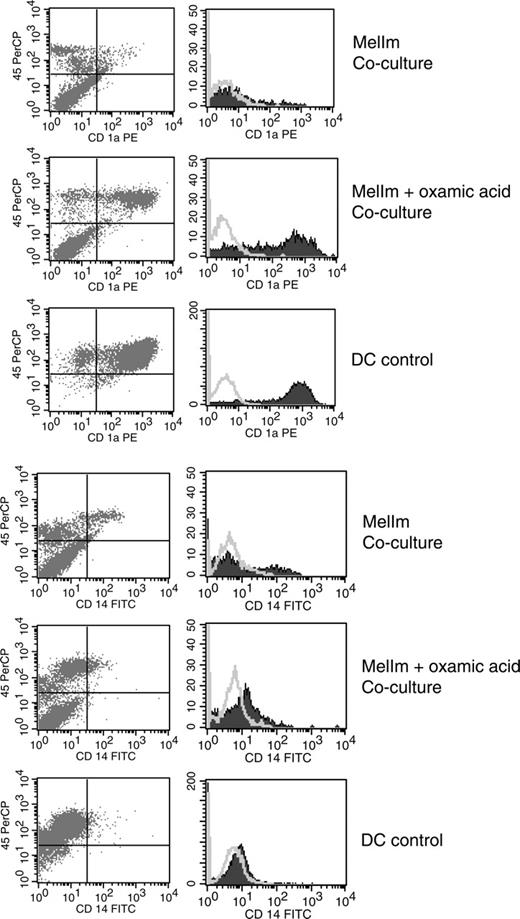
To test whether lactic acid in the tumor supernatants of MelIm is really involved in the modulation of the DC phenotype, we blocked the lactic acid production of MelIm tumor cells by the addition of oxamic acid, a well-known inhibitor of lactate dehydrogenase and used these tumor supernatants for inhibition experiments. In order to obtain high levels of lactic acid in tumor cell-conditioned media (> 15 mM), dense monolayer cultures were applied. As shown in Figure 4A, the addition of 50% MelIm supernatant to DC cultures inhibited CD1a expression. However, supernatants generated in the presence of oxamic acid contained significantly reduced lactic acid concentrations (Figure 4B) and had no effect on CD1a expression (Figure 4A). However, in the spheroid coculture other factors may be involved that are not present in the tumor supernatants. Therefore, we generated MCTSs in the presence or absence of oxamic acid and then added monocytes for DC differentiation. Figure 5 shows that CD1a expression is clearly suppressed in MelIm cocultures, whereas CD14 is increased. Blocking of lactic acid production by oxamic acid reverted the phenotype and led to an increase in CD1a expression and reduction of CD14 expression. When the same experiments were performed with J82 MCTS, only slight effects on CD1a expression and CD14 expression were found (data not shown).
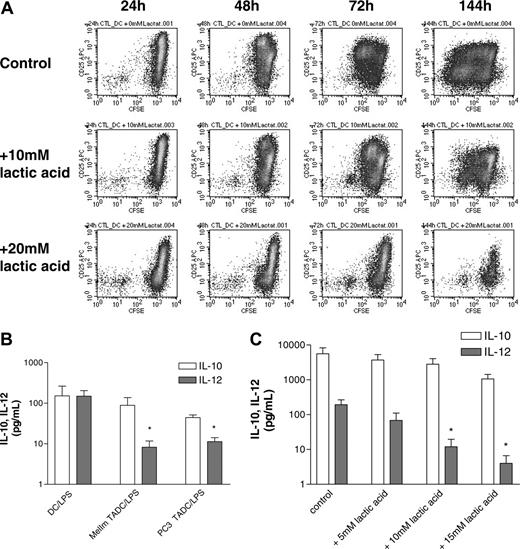
Finally, we intended to demonstrate that lactic acid also influences the functional activity of dendritic cells by adding various concentrations of lactic acid during a mixed lymphocyte reaction. A concentration-dependent inhibition of T-cell proliferation was found (data not shown). As the induction of antigen-specific T cells is a more stringent assay of DC function, we also analyzed the effect of lactic acid in such an assay. Antigen-specific CD8+ T cells were stimulated with autologous peptide-pulsed DCs, and proliferation was determined by CFSE staining in the presence or absence of lactic acid. Figure 6A clearly demonstrates that lactic acid leads to a concentration-dependent inhibition of T-cell proliferation.
To analyze the functional activity of TADCs after the MCTS coculture, we intended to separate TADCs and tumor cells by fluorescence activated cell sorting (FACS) following dissociation. Unfortunately, the separation procedure itself resulted in DC stimulation as verified with control DCs that showed an extremely high spontaneous secretion of IL-6 after FACS. We therefore analyzed IL-10 and IL-12 secretion directly in the MelIm and PC3 MCTS cocultures with DCs, as both tumor cell types alone produced no IL-10 and IL-12 in MCTS culture. When compared with control DCs, IL-10 secretion was not significantly altered in MCTS cocultures, but IL-12 levels were clearly reduced (Figure 6B). Even though we cannot be sure that the absolute cell numbers of TADCs in the coculture and the number of control DCs are the same, the ratio of IL-12 to IL-10 changes significantly when we compare control DCs (ratio, 0.98) with MelIm TADCs (0.09) or PC3 TADCs (0.25). This indicates that TADCs have a preference for IL-10 secretion. This is in accordance with the finding that control DCs stimulated with LPS showed a concentration-dependent inhibition of IL-12 secretion in the presence of lactic acid, while IL-10 levels were not significantly affected (Figure 6C). The higher level of IL-10 secretion in DCs in Figure 6C compared with Figure 6B is most likely due to different experimental conditions. In Figure 6C, immature DCs were stimulated for only 24 hours after 7 days of differentiation, whereas DCs and TADCs in Figure 6B were matured for 72 hours after 4 to 5 days of differentiation. Also, lactic acid inhibited the IL-6 and TNF-alpha production of these DCs (data not shown).
Inhibition of lactic acid production reverts the phenotypical changes induced by MelIm supernatants. Supernatants were generated in the presence or absence of oxamic acid, an inhibitor of lactate dehydrogenase. Monolayer cultures of the cell lines J82 and MelIm were grown in RPMI-1640 medium and oxamic acid was added on day 0. After 4 to 6 days, supernatants were harvested and stored at -20°C. Monocytes were cultured in the presence of 50% supernatant, and CD1a expression was determined after 7 days by flow cytometry (A). (B) Lactate in the tumor cell supernatants was determined with a routine procedure as described in “Materials and methods.” Error bars indicate SEM.
Inhibition of lactic acid production reverts the phenotypical changes induced by MelIm supernatants. Supernatants were generated in the presence or absence of oxamic acid, an inhibitor of lactate dehydrogenase. Monolayer cultures of the cell lines J82 and MelIm were grown in RPMI-1640 medium and oxamic acid was added on day 0. After 4 to 6 days, supernatants were harvested and stored at -20°C. Monocytes were cultured in the presence of 50% supernatant, and CD1a expression was determined after 7 days by flow cytometry (A). (B) Lactate in the tumor cell supernatants was determined with a routine procedure as described in “Materials and methods.” Error bars indicate SEM.
Discussion
DCs play an important role in the initiation of primary immune responses and are also implicated in antitumor activity.8,33,34 However, several authors described that the tumor milieu can influence DC differentiation.1-6 To mimic tumor-associated DC differentiation phenomena in vitro, we established a 3-dimensional model system by coculturing MCTSs with human monocytes in the presence of IL-4 and GM-CSF. Monocytes infiltrated MCTSs generated from urothelial carcinoma, prostate carcinoma, and melanoma tumor cells and differentiated into a special type of DC showing low expression of CD1a and a low secretion of IL-12.
Conflicting results have been published on the phenotype of TADCs in vivo. Thurnher et al detected mature TADCs in renal cell carcinoma,7 while high expression of HLA-DR and low expression of CD80 and CD86 on TADCs in colon carcinoma sections have been described.35 Bell et al found immature DCs within the tumor areas and mature DCs in peritumoral areas of breast carcinoma tissue.8 These data indicate that the phenotype of DCs in tumor tissue may depend on the specific tumor type, which is in line with our results.
Inhibition of lactic acid production reverts the phenotypical changes in the direct MelIm MCTS coculture. MelIm MCTSs were generated in the presence or absence of oxamic acid. To exclude the possibility that oxamic acid influences DC differentiation directly, MCTSs were washed before adding the monocyte suspension. After 5 to 7 days in coculture, MCTSs were dissociated by vigorous pipetting and stained with mouse anti-human CD45 antibody in combination with antibodies against CD1a and CD14. The same staining procedure was performed with control DCs. Histograms represent CD45+ cells. The figure shows 1 representative experiment of 3. Open histograms represent isotype controls; filled histograms, specific staining.
Inhibition of lactic acid production reverts the phenotypical changes in the direct MelIm MCTS coculture. MelIm MCTSs were generated in the presence or absence of oxamic acid. To exclude the possibility that oxamic acid influences DC differentiation directly, MCTSs were washed before adding the monocyte suspension. After 5 to 7 days in coculture, MCTSs were dissociated by vigorous pipetting and stained with mouse anti-human CD45 antibody in combination with antibodies against CD1a and CD14. The same staining procedure was performed with control DCs. Histograms represent CD45+ cells. The figure shows 1 representative experiment of 3. Open histograms represent isotype controls; filled histograms, specific staining.
Lactic acid inhibits DC activation during antigen-specific autologous T-cell stimulation. (A) Purified CD8+ T cells were labeled with CFSE prior to stimulation with peptide-pulsed DCs in the absence or presence of lactic acid. After 1 to 6 days, T cells were stained with anti-CD3 and anti-CD25. For flow cytometric analysis, CD3+ cells were gated and analyzed after 24 hours up to 144 hours. (B) TADCs show an impaired IL-12 secretion compared with control DCs. MelIm-MCTSs and PC3-MCTSs were generated using the liquid overlay culture technique. On day 5, half of the medium was replaced with a monocyte suspension. After another 4 to 5 days, MelIm-TADC and PC3-TADC cocultures were matured for 72 hours with 10 ng/mL LPS. Control DCs were also matured for 72 hours. Supernatants were harvested and stored at -20°C. IL-10 and IL-12 (p70) were determined in the culture supernatants with commercially available ELISA kits (*P < .05). (C) Lactic acid inhibits LPS-stimulated cytokine secretion by control DCs. DCs were generated in RPMI-1640 supplemented with GM-CSF and IL-4 and harvested on day 7 of culture. DCs (1 × 106/2 mL) were transferred into 6-well plates and stimulated with 100 ng/mL LPS in the absence or presence of various concentrations of lactic acid. After 24 hours, supernatants were harvested, filtered, and stored at -20°C. IL-10 and IL-12 (p70) were determined in the culture supernatants with commercially available ELISA kits (*P < .05). (B-C) Error bars indicate SEM.
Lactic acid inhibits DC activation during antigen-specific autologous T-cell stimulation. (A) Purified CD8+ T cells were labeled with CFSE prior to stimulation with peptide-pulsed DCs in the absence or presence of lactic acid. After 1 to 6 days, T cells were stained with anti-CD3 and anti-CD25. For flow cytometric analysis, CD3+ cells were gated and analyzed after 24 hours up to 144 hours. (B) TADCs show an impaired IL-12 secretion compared with control DCs. MelIm-MCTSs and PC3-MCTSs were generated using the liquid overlay culture technique. On day 5, half of the medium was replaced with a monocyte suspension. After another 4 to 5 days, MelIm-TADC and PC3-TADC cocultures were matured for 72 hours with 10 ng/mL LPS. Control DCs were also matured for 72 hours. Supernatants were harvested and stored at -20°C. IL-10 and IL-12 (p70) were determined in the culture supernatants with commercially available ELISA kits (*P < .05). (C) Lactic acid inhibits LPS-stimulated cytokine secretion by control DCs. DCs were generated in RPMI-1640 supplemented with GM-CSF and IL-4 and harvested on day 7 of culture. DCs (1 × 106/2 mL) were transferred into 6-well plates and stimulated with 100 ng/mL LPS in the absence or presence of various concentrations of lactic acid. After 24 hours, supernatants were harvested, filtered, and stored at -20°C. IL-10 and IL-12 (p70) were determined in the culture supernatants with commercially available ELISA kits (*P < .05). (B-C) Error bars indicate SEM.
In vitro data suggest that soluble factors such as IL-10, VEGF, IL-6, and M-CSF are implicated in tumor-associated DC modulation.13,14,36,37 In our experimental system, tumor cell supernatants could partially replace the direct coculture system and similar to Menetrier-Caux et al13 we found IL-6 and M-CSF at least in the supernatant of urothelial carcinoma MCTSs.
In contrast to the urothelial carcinoma cell line J82, which secreted high amounts of cytokines, melanoma cell lines produced no IL-6, M-CSF, VEGF, or IL-10, indicating additional mechanisms of suppression. Berthier-Vergnes et al investigated the modulation of DC (Langerhans cell) differentiation after coculture with melanoma cells and excluded an impact of TGF beta, IL-10, and VEGF in their system.38 According to these data and our own observations, alternative DC modulating molecules are to be hypothesized. Here, it is to be considered that the tumor milieu is determined not only by cytokines but also by low oxygen tension (hypoxia, low pO2),39,40 increased CO2 pressure, lactic acid production, and accumulation and the generation of a low interstitial pH.41,42
Hypoxia, for example, was found to influence monocytic cells with respect to morphology, antigen expression, and cytokine secretion,23,43 and to induce various genes including VEGF, LDH, and MMPs.21,44-46 However, moderate hypoxia alone did not induce a TADC phenotype comparable with that found in the melanoma MCTS culture system with respect to marker expression and T-cell stimulation.
In contrast to low oxygen tensions, addition of lactic acid in concentrations found in tumor spheroids47 and tumor biopsies (10 mM or even higher)48,49 induced a modulation of the DC phenotype comparable with melanoma cell supernatants (eg, down-regulation of CD1a and up-regulation of CD86). Ligation of B7-1/B7-2 has been shown to trigger indoleamine 2,3-dioxygenase activity in DCs and in turn inhibits the T-cell response.50 In light of this publication, one could speculate that the up-regulation of CD86 on TADCs may be involved in the inhibition of T-cell activity. The up-regulation of CD86 may also indicate a differentiation toward a macrophage-like phenotype because this antigen is also strongly expressed on macrophages. In line with this hypothesis, TADCs also expressed CD14, a classical monocyte-macrophage marker.
Puig-Kröger et al described that peritoneal dialysis solutions containing lactate and glucose-degradation products inhibit the differentiation and maturation of monocyte-derived DCs.51 However, inhibitory effects on DCs were especially found with high-lactate-containing solutions (40 mM lactate) and were dependent on glucose-degradation products. In contrast to our findings, the sodium salt of lactic acid, lactate, suppressed CD1a expression in their experiments and this effect was independent of the pH. At physiologic pH, lactic acid almost entirely dissociates to the lactate anion, which cannot cross the plasma membrane by free diffusion. It requires a specific transport system provided by the proton-linked monocarboxylate transporters (MCTs), which cotransport protons and lactate anion.32 The presence of MCT1, 2, and 4 has been demonstrated in lymphocytes, granulocytes, and monocytes.52 A pH gradient is necessary to facilitate the transport of lactate into a cell; thus, a low extracellular pH leads to an enhanced accumulation of lactate in macrophages.53 In our system, the effect of 10 mM lactic acid on DC differentiation was pH dependent. We therefore assume that the effect of lactic acid depends on the lactate transport system, whereas other mechanisms may apply for higher sodium lactate concentrations. Accordingly, the inhibitory effect of 15 mM lactic acid could only partially be reversed by pH adjustment, which correlates with the study by Puig-Kröger et al51 showing 15 mM to 40 mM lactate being effective at pH 7.4.
Lactate concentrations of 10 mM and higher are found under various pathophysiologic conditions (eg, in dialysis solutions, under inflammatory conditions, and especially in tumor tissues). High lactate concentrations in tumor biopsies correlate with the incidence of metastasis and a reduction in patient survival,48,54 suggesting that the expression of LDH and the production of lactic acid are a growth advantage for tumor cells and/or may contribute to escape the immune surveillance. In our hands, lactic acid not only altered the antigen expression of DCs under various culture conditions (immature/mature DCs, Langerhans cells) but also strongly inhibited antigen presentation in an allogeneic and autologous experimental setting. In addition, IL-12 secretion was significantly reduced in TADCs in MCTS cocultures. A similar reduction was found in control DCs supplemented with lactic acid during the activation process. This situation may apply for lymph nodes with tumor metastases where antigen presentation would be suppressed even when DCs are not premodulated. Ongoing studies in our laboratory will verify if other immune cells beside DCs (ie, lymphocytes) are targets of the immune modulation by lactic acid during antigen presentation, although previous reports indicate a positive effect of sodium lactate on IL-2 expression in T cells.55,56
In summary, we conclude that various tumor-derived factors modulate the phenotype of infiltrating monocytic cells. These factors include not only the cytokine pattern (eg, IL-6 and M-CSF) but also products of tumor metabolism such as lactic acid. Thus, high lactic acid concentrations found in tumor tissues can alone or in combination with cytokines induce a tumor-specific type of DCs.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2005-05-1795.
Supported by Deutsche Forschungsgemeinschaft KR 1418/6-1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Antigen expression of TADCs cultured under different conditions. The urothelial carcinoma cell lines J82 and UMUC3 and the melanoma cell lines MelIm and Mel108 were used for MCTS generation. After 4 to 5 days, half of the medium was replaced by a monocyte suspension in IL-4 and GM-CSF (A) or in IL-4, GM-CSF, and TGF beta 1 (B). In some experiments, LPS was added during the last 72 hours to induce DC maturation (B). After 5 to 7 days in coculture, MCTSs were dissociated and stained with mouse anti-human CD45 antibody in combination with antibodies against the indicated antigens. The same staining procedure was performed with control DCs. For flow cytometry analysis, CD45+ cells were gated. Panel A shows the mean and SEM of the median fluorescence intensity (isotype subtracted) of at least 3 experiments (CD1a expression: control DCs versus J82 TADCs [P < .001], control DCs vs UMUC3 TADCs/Mel108 TADCs/MelIm TADCs [P < .05], unpaired t test). Panel B represents 1 representative experiment of 3. Quadrant statistics are shown for the top right quadrant (CD45+ cells were gated and represent 100%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/5/10.1182_blood-2005-05-1795/2/m_zh80050692210002.jpeg?Expires=1767746665&Signature=uKcIe9EIUS4L~5abWQEfB3c7fIlyXnzdP6hmp5A5cCTDDZhroNOGogoSsD~dOomlxQoC9n1ctieipV7a2wenjbn2E9z9LC1xJhtLzCSt-Ios-dHZXG3D~Ix6nMbQwwqa5zyxR5qBB4JTpu0qouxa504VOqUZP4ROfgsAO3VluvDZ15xngC8DFmJy30rI35nnJh1xQwk-F~aDaW0XqG9j7fDfa0kLNnv~-g2vuuO9ztEvbcxVkbG~X5kQ0Pn-6aV8440WTAQJtjjCR-s1SUBYKXHhI5ETagdh2l96CU~3Y8Yk554H63c03KYfBqjxWN37Y09i~oCh02KEEV64Txbf7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal