The mechanisms regulating thymic involution are unclear. In inbred mouse strains the rate of thymic involution and the function of the hematopoietic stem cell (HSC) compartment are subject to quantitative genetic variation. We have shown previously that transforming growth factor-β2 (TGF-β2) is a genetically determined positive regulator of HSCs. Here, we demonstrate that genetic variation in the rate of thymic involution correlates with genetic variation in the responsiveness of hematopoietic stem and progenitor cells to TGF-β2. Corroborating these correlations, thymic cellularity and peripheral naive T-cell frequency were higher in old Tgfb2+/- mice than in wild-type littermates. The frequency of early T-cell precursors was increased in Tgfb2+/- mice, suggesting that TGF-β2 affects the earliest stages of T-cell development in old mice. Reciprocal transplantation experiments indicated that TGF-β2 expressed both in the (micro)environment and in the hematopoietic system can accelerate thymic involution; however, the age of the stem cells appeared irrelevant. Thus, although thymic involution is largely determined by the aged environment, TGF-β2 plays a major modulatory role that is subject to genetic variation and is possibly mediated through its regulatory effects on early hematopoiesis.
Introduction
The development of T cells occurs in the thymus from hematopoietic cells that seed this organ from the liver during fetal life and from the bone marrow after birth.1 With age, the thymus involutes, leading to a progressive decline in the production of naive T cells.2-4 The mechanism and purpose of thymic involution remain a mystery. The timing of the onset of this process, just before and during adolescence, as well as some experimental evidence suggest that endocrine hormones may be involved.4-6 Age-related changes in the thymic microenvironment, especially a decreased production of interleukin 7 (IL-7) have also been proposed to play a role,7,8 although overexpression of IL-7 within the thymus did not restore thymic function in aged mice.9 IL-12 is involved in partially maintaining thymic function in aged animals by enhancing the response of thymocytes to IL-2 and IL-7.10 A differentiation block between the CD4-CD8-CD44+CD25- (double-negative 1, DN1) and CD4-CD8-CD44+CD25+ (double-negative 2, DN2) stages of the early intrathymic T-cell development has been observed and was believed to be critical for the decline in T-cell production in the involuting thymus.11 However, early T-cell precursors (ETPs), a lineage negative (lin-) CD25-c-kit+IL7Rα-/lo population that has recently been shown to contain the earliest intrathymic T-cell precursors,12 do not accumulate with age.13 Because ETPs make up only a small fraction of the heterogeneous DN1 population,14 the nature of the accumulating DN1 cells in the involuting thymus is unclear. A role for hematopoietic stem cells (HSCs) and age-related changes in the function of these cells, particularly in their capacity to differentiate into the lymphoid lineage,15-18 is controversial,2-4 because transplantation with HSCs from young mice does not rejuvenate the thymus.19
Extensive mouse strain-dependent variation has been demonstrated in the rate of thymic involution, and suggestive quantitative trait loci (QTLs) for this multigenic trait have been mapped to regions on chromosomes 9 and 10.20 Quantitative genetic variation also exists in the hematopoietic stem and progenitor cell compartment.21 The frequency of hematopoietic stem and progenitor cells, as determined by the lin-Sca1++c-kit+ (LSK) phenotype,22 shows wide mouse strain-dependent variation and is regulated in part by genetic variation in the signaling of one particular transforming growth factor-β (TGF-β) isoform, TGF-β2.23-25 In contrast to the presumed inhibitory role of other TGF-β isoforms, TGF-β2 is a positive regulator of HSC number and function in vivo.25
Here, we demonstrate that genetic variation in the rate of thymic involution, as determined by Hsu et al,20 correlates with genetic variation in the responsiveness of hematopoietic stem and progenitor cells to TGF-β2, as previously reported by us.25 Further studies revealed that, although thymic involution is to a large extent determined by the aging environment, TGF-β2 accelerates thymic involution in a genetically determined fashion, an effect that is potentially mediated through its regulatory role on early hematopoiesis.
Materials and methods
Mice
Eight-week-old C57BL/6J, DBA/2J, BXD recombinant inbred (RI) and heterozygous tgfb2tm1doe mice (Tgfb2-/- mice die at birth26 ) were purchased from Jackson Laboratories (Bar Harbor, ME). C57BL/6.SJL-PtprcaPep3b/BoyJ mice were purchased from the National Cancer Institute (Bethesda, MD). Animals were kept in a specific pathogen-free facility. Experiments and animal care were performed in accordance with the Mount Sinai Institutional Animal Care and Use Committee (IACUC).
Antibodies and flow cytometry
Unlabeled CD2, CD3, CD8α, CD4, B220, Ly6G/Gr1, Mac1, FITC-conjugated CD45.1, CD8α, CD4, B220, Gr1, Mac1, CD45.2, and goat anti–rat immunoglobulin, and PE-conjugated CD45RB were purchased from Southern Biotechnologies (Birmingham, AL). Unconjugated Ter119, FITC-conjugated CD8β, anti–T-cell receptor γδ (anti-TCRγδ, and CD25, PE-conjugated anti-ILRα, CD25 and Sca1, APC-conjugated CD44 and anti–c-kit, biotinylated anti–c-kit, and APC-Cy7–conjugated CD8, CD19, and streptavidin were purchased from Pharmingen (San Diego, CA). FITC-conjugated anti-TCRβ, CD3ϵ, Ter119, and NK1.1 were obtained from eBiosciences (San Diego, CA).
Thymus
Thymi were dissected from humanely killed mice, weighed, and minced through a nylon mesh. Mononuclear cells were counted using a hemocytometer after gradient centrifugation using lymphocyte separation medium. More than 98% of the cells were Thy1+. Thymocytes were labeled with various combinations of antibodies and analyzed on an LSR II multilaser flow cytometer with Diva software (Becton Dickinson, Mountain View, CA).
Bone marrow transplantation
In reciprocal transplantation experiments 2 × 106 bone marrow cells from CD45.2+CD45.1+ heterozygous C57BL/6.SJL-PtprcaPep3b/BoyJTgfb2+/- or C57BL/6.SJL-PtprcaPep3b/BoyJ wild-type (wt) F1 mice were injected into lethally (950 cG) irradiated C57BL/6 mice (CD45.2+) or vice versa. Peripheral blood, bone marrow, spleen, and thymus were harvested after 12 months and analyzed for the expression of CD45.1 and CD45.2 to evaluate the level of donor-derived reconstitution. Reconstitution was more than 90% in myeloid, B, and T lineages in all mice (not shown). The thymi were processed as described (see “Thymus”).
Real-time PCR
Total RNA was isolated from thymi using TRIzol reagent (Gibco, Grand Island, NY) according to the manufacturer's instructions and treated with DNaseI. RNA was reverse transcribed using Superscript III (Gibco), for 1 hour at 42°C using oligo(dT) primers. Primers for IL-7 were TGCTTTTTCCAGCCACGTGA (sense) and CAAGAAGGCATGGCTACCAC (antisense). Real-time polymerase chain reaction (PCR) was performed by the Mount Sinai Real-Time PCR Shared Resource on a TaqMan with detection using SYBR Green. Standards were β-actin, rps11, and α-tubulin. Each sample was run in triplicate. Relative amounts of mRNA were assessed by comparing the crossing threshold for IL-7 with the corrected median value of the 3 housekeeping genes.
Histology
Fixed (phosphate-buffered formalin, pH 7.0) thymi were embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin. Surface areas of cortex and medulla were calculated using ImageJ (available at http://rsb.info.nih.gov/ij/download.html) after manual demarcation on TIF files generated using a Leica MZFL 3 stereo dissecting microscope equipped with a Plan Apo objective lens and a 12.5:1 zoom magnification changer, which was used at an approximate magnification of 1 to 1.5 (Leica, Wetzlar, Germany). Images were acquired using an Optronics Magnafire camera and Magnafire software version 2.0 (Optronics, Goleta, CA).
Statistics
The 2-tailed Student t test for paired samples was used in all comparisons of Tgfb2+/- mice and wt littermates. The 2-tailed Student t test for unpaired samples was used in the transplantation experiments. All results are expressed as mean plus or minus standard error of the mean (SEM). P value below .05 was considered indicative of a statistically significant difference.
Results
Recombinant inbred (RI) mouse strains are a powerful tool to investigate quantitative genetic variation in the mouse and to map QTLs. BXD RI strains are commercially available and were generated by repeated inbreeding of F2 mice derived from the inbred progenitor strains, C57BL/6 and DBA/2. The genome of RI strains is composed of a patchwork of homozygous chromosome segments derived from either progenitor strain, with each of the RI lines having a unique combination of “patches” from the progenitors. As a consequence of the homozygous “reshuffling” of C57BL/6 and DBA/2 alleles, RI strains will show a continuous range of values for complex or multigenic traits, with some BXD RI mice having more extreme phenotypes than the 2 progenitor strains, a phenomenon called transgression.27
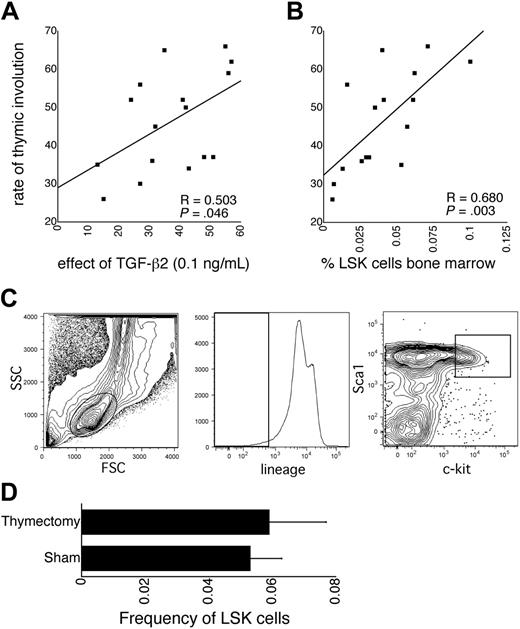
Among BXD RI strains, the rate of thymic involution shows wide variation, although it is similar in the progenitors, C57BL/6 and DBA/2.20 Several traits related to the hematopoietic stem and progenitor cell compartment are also subject to mouse strain–dependent genetic variation.21,23-25 We have previously shown that one mechanism underlying this genetic variation is TGF-β2 signaling.25 In vitro, the TGF-β2 dose response on the proliferation of LSK cells is biphasic. It is composed of a serum-dependent stimulatory component at low concentrations that is subject to genetic variation and a serum-independent inhibitory component at higher concentrations that is not subject to genetic variation.25,28 This biphasic dose response is specific for LSK cells and for TGF-β2, as other TGF-β isoforms are only inhibitors of LSK cell proliferation.25 Mouse strain-dependent variation in the TGF-β2 dose response maps to a QTL on chromosome 4, overlapping with a QTL contributing to LSK cell frequency. Adult heterozygous Tgfb2+/- mice have an HSC defect compared with wt littermates. These findings demonstrate that TGF-β2 is a genetically determined positive regulator of HSCs and suggest that the stimulatory effect on LSK cell proliferation at low concentrations of this factor is relevant in vivo.25 We found that among the 16 BXD RI strains for which all data were available, the effect of TGF-β2 at 0.1 ng/mL on the proliferation of LSK cells from 8-week-old mice supported by early-acting cytokines25 correlated significantly with the rate of thymic involution (Hsu et al20 and http://www.webQTL.org; Figure 1A). The rationale for using TGF-β2 at 0.1 ng/mL was that at this concentration, where the net effect on LSK proliferation is, in fact, inhibitory, the mouse strain–dependent variation in the TGF-β2 dose response was the most pronounced.25 These data suggest a role for TGF-β2 in the regulation of thymic involution and indicate that approximately 25% of the genetically determined variation in the rate of thymic involution may be explained by quantitative variation in the TGF-β2 responsiveness of LSK cells. That no overlapping QTLs were identified for these 2 traits may be explained by the fact that all QTLs were suggestive and by the relatively small number (18) of BXD RI strains included in the analysis of Hsu et al,20 increasing the probability of false map locations.27 LSK cell frequency in BXD RI mice also correlated with thymic involution (Figure 1B,C). This is not unexpected because TGF-β2 regulates the frequency of LSK cells in vivo.25 The correlation between the rate of thymic involution and the frequency of LSK cells may also suggest that thymic involution per se increases the frequency of LSK cells, however. To investigate this possibility, LSK cell frequency was measured 5 months after neonatal thymectomy. Neonatal thymectomy did not affect the frequency of LSK cells (Figure 1D). Although the consequences of neonatal thymectomy are not necessarily the same as those of thymic involution, it is unlikely that loss of functional thymic tissue regulates LSK cell frequency.
Correlations between hematopoietic traits and thymic involution. (A) Correlation between the rate of thymic involution (from Hsu et al20 and available on http://www.webQTL.org) and the proliferative response of LSK cells to TGF-β2 (0.1 ng/mL) in liquid cultures supported by kit ligand, flt3 ligand, and thrombopoietin, expressed as a percent of control cultures without TGF-β2 (from Langer et al25 ). Note that at this concentration in the biphasic TGF-β2 dose response, the net effect is in fact inhibitory (Langer et al25 ). The difference in the effect of TGF-β2 among BXD mice was largest at that concentration, however. (B) Correlation between the rate of thymic involution and the frequency of LSK cells in the bone marrow (from Henckaerts et al24 ). (C) Sort windows used for the analysis of the frequency of LSK cells in the bone marrow of BXD RI mouse strains. (D) Frequency of LSK cells in bone marrow 5 months after neonatal (day 7) thymectomy in C57BL/6 mice (n = 5).
Correlations between hematopoietic traits and thymic involution. (A) Correlation between the rate of thymic involution (from Hsu et al20 and available on http://www.webQTL.org) and the proliferative response of LSK cells to TGF-β2 (0.1 ng/mL) in liquid cultures supported by kit ligand, flt3 ligand, and thrombopoietin, expressed as a percent of control cultures without TGF-β2 (from Langer et al25 ). Note that at this concentration in the biphasic TGF-β2 dose response, the net effect is in fact inhibitory (Langer et al25 ). The difference in the effect of TGF-β2 among BXD mice was largest at that concentration, however. (B) Correlation between the rate of thymic involution and the frequency of LSK cells in the bone marrow (from Henckaerts et al24 ). (C) Sort windows used for the analysis of the frequency of LSK cells in the bone marrow of BXD RI mouse strains. (D) Frequency of LSK cells in bone marrow 5 months after neonatal (day 7) thymectomy in C57BL/6 mice (n = 5).
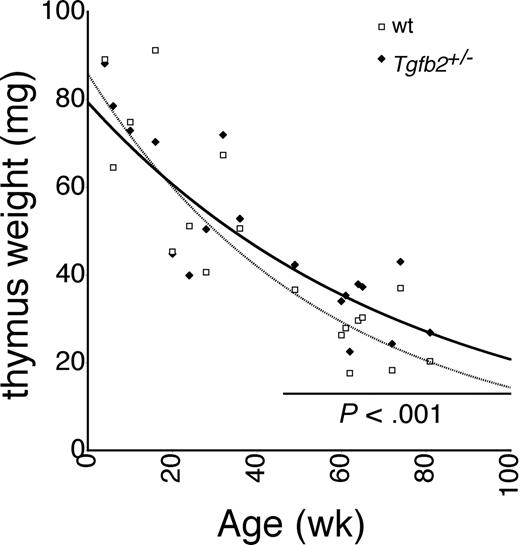
Because the correlation data in BXD RI strains suggested a role for TGF-β2 in thymic involution, we analyzed thymic involution in Tgfb2+/- mice (Tgfb2-/- mice die perinatally26 ). Tgfb2+/- mice were backcrossed onto the C57BL/6 background for 11 generations. No difference was observed between young (< 12 months) Tgfb2+/- and wt mice. However, in older mice the involution curves began to diverge, and older than age 12 months, thymus weight was significantly higher in Tgfb2+/- mice than in wt mice (Figure 2). Similar data were obtained when the ratio of thymus weight to body weight was calculated (not shown). Thymus cellularity paralleled thymus weight, and was 29% ± 11% higher in aged Tgfb2+/- mice than in wt mice, although variability was larger (P = .05, not shown). More than 98% of the low-density cells were Thy1+. Taking into account that Thy1 is not expressed in the earliest phases of T-cell development, virtually all low density cells used in the cell counts were thymocytes. The larger size and cellularity of the thymus in aged Tgfb2+/- mice were therefore mainly due to increased numbers of thymocytes. No difference was observed in the body weight of Tgfb2+/- and wt mice at any age (not shown, n = 44 for Tgfb2+/- mice and n = 63 for wt littermates); hence, differences in body weight cannot explain the observed difference in size and cellularity of the thymus. Thus, the lower rate of thymic involution in Tgfb2+/- mice demonstrates that TGF-β2 contributes to thymic involution, corroborating the correlation data shown in Figure 1.
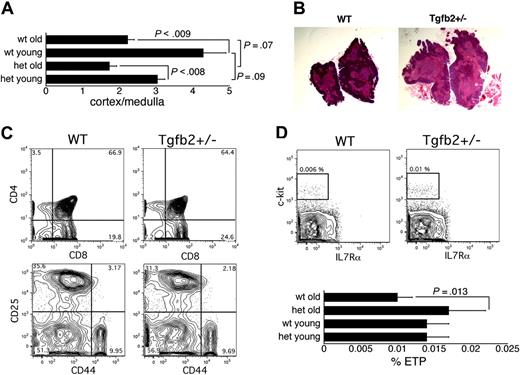
Thymic involution is accompanied by a decrease in the ratio of cortical to medullary surface area or thickness.2-4,20 Histologic analysis revealed that the age-related decrease in the ratio of cortical to medullary surface area was similar in wt and Tgfb2+/- thymi (Figure 3A). Somewhat surprisingly, the corticomedullary ratio appeared lower at any age in Tgfb2+/- than in wt mice, although the difference just failed to reach statistical significance (Figure 3A,B). However, when data for old and young mice were pooled, the difference was statistically significant (P = .04). Thus, TGF-β2 deficiency slightly decreased the ratio of cortical to medullary surface area, but did not affect the age-related changes in this parameter.
Because thymic involution has been attributed to an age-related decrease in the production of IL-7,7 we measured IL-7 mRNA levels in thymi of aged Tgfb2+/- mice and wt littermates by real-time PCR. No difference in IL-7 mRNA expression was observed (not shown), indicating that TGF-β2 does not affect IL-7 production in the thymus. We next analyzed the thymi of Tgfb2+/- mice and wt littermates by flow cytometry. Staining for CD4 and CD8 revealed similar proportions of single-positive, double-positive, and double-negative cells in aged Tgfb2+/- and wt mice (Figure 3C). Analysis of early thymopoiesis, as measured by the expression of CD25 and CD44 on triple-negative (TN) cells,29 defined as cells negative for CD3, CD4, CD8α, CD8β, and non–T-lineage antigens (NK1.1, B220, Ter119, Gr-1, Mac1), showed similar frequencies of TN1, TN2, TN3, and TN4 cells in aged Tgfb2+/- and wt mice (Figure 3C). However, the frequency of ETPs, defined as lin-CD25-c-kit+ILR7α-/lo cells12 (lineage markers were CD3, CD8α, CD8β, TCRαβ, TCRγδ, NK1.1, CD19, Ter119, B220, Mac1, Gr1), was higher in the thymi of aged Tgfb2+/- mice than of aged wt mice. In contrast, ETP frequency was similar in young Tgfb2+/- and wt mice (Figure 3D). Taken together, our data show that in the thymi of aged Tgfb2+/- mice, except for the earliest detectable T-cell precursors, all thymocyte populations appeared to be increased proportionally compared with wt thymi. Thus, the larger thymi of aged Tgfb2+/- mice cannot be attributed to a specific or malignant subpopulation. Rather, TGF-β2 appeared to affect the earliest stage of T-cell development in old mice.
Thymic involution in Tgfb2+/- mice. Thymus weight in Tgfb2+/- mice and wt littermates at various ages. Each pair of data points represents the average of 2 to 4 Tgfb2+/- and wt members of a litter.
Thymic involution in Tgfb2+/- mice. Thymus weight in Tgfb2+/- mice and wt littermates at various ages. Each pair of data points represents the average of 2 to 4 Tgfb2+/- and wt members of a litter.
Analysis of the involuting thymus in Tgfb2+/- and wt mice. (A) Ratio of cortical to medullary surface area in Tgfb2+/- (het) and wt mice (n = 12 sections from 3 thymi; young are 8 weeks old, old are 14-16 months old). (B) Representative example of hematoxylin and eosin-stained thymi from 16-month-old wt and Tgfb2+/- mice (original magnification, × 10). (C) Representative example of staining of thymi from wt and Tgfb2+/- mice for CD4 and CD8 (top panels) and for CD25 and CD44 (bottom panels, gated on cells negative for CD3, CD4, CD8α, CD8β, B220, Ter119, NK1.1, Gr-1, Mac1). (D) Representative example of the detection of ETPs (lin- CD25-c-kit+IL7Rα-/lo, plots gated on cells that were negative for CD25, CD3, CD8α, CD8β, TCRαβ, TCRγδ, NK1.1, CD19, Mac1, GR1, B220 and Ter119) in wt and Tgfb2+/- thymi (top panels), and frequency of ETPs in thymi from young (2-6 months) and old (14-16 months) Tgfb2+/- mice and wt littermates (n = 4 litters in young mice and 5 litters in old mice; 2 mice from each genotype and each litter were pooled for each analysis).
Analysis of the involuting thymus in Tgfb2+/- and wt mice. (A) Ratio of cortical to medullary surface area in Tgfb2+/- (het) and wt mice (n = 12 sections from 3 thymi; young are 8 weeks old, old are 14-16 months old). (B) Representative example of hematoxylin and eosin-stained thymi from 16-month-old wt and Tgfb2+/- mice (original magnification, × 10). (C) Representative example of staining of thymi from wt and Tgfb2+/- mice for CD4 and CD8 (top panels) and for CD25 and CD44 (bottom panels, gated on cells negative for CD3, CD4, CD8α, CD8β, B220, Ter119, NK1.1, Gr-1, Mac1). (D) Representative example of the detection of ETPs (lin- CD25-c-kit+IL7Rα-/lo, plots gated on cells that were negative for CD25, CD3, CD8α, CD8β, TCRαβ, TCRγδ, NK1.1, CD19, Mac1, GR1, B220 and Ter119) in wt and Tgfb2+/- thymi (top panels), and frequency of ETPs in thymi from young (2-6 months) and old (14-16 months) Tgfb2+/- mice and wt littermates (n = 4 litters in young mice and 5 litters in old mice; 2 mice from each genotype and each litter were pooled for each analysis).
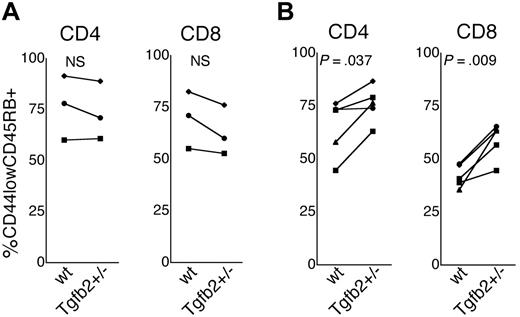
Thymic involution leads to a decreased production of naive T cells and a concomitant expansion of memory T cells.2-4,30,31 We therefore compared the fraction of naive (CD44lowCD45RBhigh)32 CD4 and CD8 cells in the peripheral blood and in the spleen of 8-week-old and 12-month-old Tgfb2+/- and wt mice. In 8-week-old mice, the frequency of naive cells (expressed as a fraction of the total CD4 or CD8 population) was similar in Tgfb2+/- and wt mice (Figure 4A). In contrast, 12-month-old wt mice had significantly lower frequencies of naive CD4 and CD8 cells in the spleen (not shown) and the peripheral blood (Figure 4B) than Tgfb2+/- mice. These data strongly suggest that the difference in the size of the thymus between old Tgfb2+/- mice and wt littermates is biologically significant.
The unique positive regulatory effect of TGF-β2 on HSC number and function is at least in part cell autonomous, because even in a wt environment, Tgfb2+/- HSCs cycle more slowly than wt HSCs.25 To examine the contribution to thymic involution of TGF-β2 expressed within the hematopoietic system as opposed to outside the hematopoietic system, we performed transplantations in 8-week-old wt and Tgfb2+/- mice, which have a similar thymus size at that age, with 2 × 106 wt or Tgfb2+/- bone marrow cells from 8-week-old donors, and measured thymus size after 12 to 14 months. In all mice, more than 90% of cells in hematopoietic organs were donor-derived at the time of analysis (not shown). Thymus size was measured as the number of thymic mononuclear cells, because dissection of thymi from irradiated animals was technically more challenging, making assessment of thymic weight less reliable. The highest thymic cellularity was observed in Tgfb2+/- recipients of Tgfb2+/- bone marrow (het→het; Figure 5A). Compared with het→het mice, thymic cellularity was decreased to the same extent in wt→wt, wt→het, and het→wt mice (Figure 5A). These data suggest that an increased expression level of TGF-β2 either within the hematopoietic system or in nonhematopoietic tissues was sufficient to accelerate thymic involution in Tgfb2+/- mice to the level of wt mice. The frequency of naive T cells in the peripheral blood correlated with thymic cellularity (Figure 5B). These data again strongly suggest that variation in thymic involution in mice given transplants has a repercussion on the composition of the peripheral T-cell pool.
Naive T cells in Tgfb2+/- mice. Fraction of naive (CD44lowCD45RB+) cells of the CD4 and CD8 populations in the peripheral blood of 8-week-old (A) and 12-month-old (B) Tgfb2+/- mice and wt littermates. Each connected pair of data points represents the average of 2 to 4 Tgfb2+/- and wt members of a litter.
Naive T cells in Tgfb2+/- mice. Fraction of naive (CD44lowCD45RB+) cells of the CD4 and CD8 populations in the peripheral blood of 8-week-old (A) and 12-month-old (B) Tgfb2+/- mice and wt littermates. Each connected pair of data points represents the average of 2 to 4 Tgfb2+/- and wt members of a litter.
Tgfb2+/- HSCs cycle more slowly in vivo.25 It is possible that the lower cycling activity of Tgfb2+/- HSCs delays age-related changes in HSCs, which contribute to thymic involution. Therefore, we examined whether thymic involution would be accelerated after reconstitution with aged as compared with young bone marrow cells. The 8-week-old CD45.2+ C57BL/6 mice were reconstituted with bone marrow cells from either 8-week-old or 18-month-old CD45.1+ C57BL/6 mice. Thymic cellularity was examined after 12 months. Mice reconstituted with bone marrow cells from young mice had a slightly higher thymic cellularity than mice reconstituted with bone marrow cells from old mice, but the difference was not significant (P = .5; Figure 5C). Furthermore, there was no difference in the frequency of naive T cells between mice reconstituted with old or young bone marrow cells (not shown). These data indicate that the aged environment in the recipient plays an important role in thymic involution.
Effect of HSC genotype and age on thymic involution. (A) Thymic cellularity 12 months after reciprocal transplants between wt and Tgfb2+/- (het) mice (mean ± SEM, n = 3-5 mice from 3 separate transplantation experiments; *significantly different from het→het transplants). (B) Correlation between the fraction of naive (CD44lowCD45RB+) CD4 cells in the peripheral blood and thymic cellularity in the transplant recipients of panel A for which data were available (n = 18). (C) Effect of the age of reconstituting bone marrow cells on thymic cellularity 12 months after transplantation (mean ± SEM; n = 10 for recipients of bone marrow of aged mice; n = 7 for recipients of bone marrow from young mice).
Effect of HSC genotype and age on thymic involution. (A) Thymic cellularity 12 months after reciprocal transplants between wt and Tgfb2+/- (het) mice (mean ± SEM, n = 3-5 mice from 3 separate transplantation experiments; *significantly different from het→het transplants). (B) Correlation between the fraction of naive (CD44lowCD45RB+) CD4 cells in the peripheral blood and thymic cellularity in the transplant recipients of panel A for which data were available (n = 18). (C) Effect of the age of reconstituting bone marrow cells on thymic cellularity 12 months after transplantation (mean ± SEM; n = 10 for recipients of bone marrow of aged mice; n = 7 for recipients of bone marrow from young mice).
Discussion
Mechanistic insight into the process of thymic involution is largely lacking.2-4 Here, we identify TGF-β2 as a factor contributing to the late stages of thymic involution and show that genetic variation in isoform-specific TGF-β2 signaling plays a major role in the quantitative genetic variation in the rate of thymic involution in inbred mice.
It is unclear whether TGF-β2 acts on the thymic microenvironment or on the hematopoietic system to regulate thymic involution. TGF-β2 may, in fact, have multiple and opposing effects on thymic function. The lower ratio between cortical and medullary surface area in Tgfb2+/-mice, generally considered a sign of architectural disintegration accompanying aging,2,3,20 may suggest that higher levels of TGF-β2 expression are beneficial for the integrity of the thymic microenvironment. The significance of this finding is unclear, however, because, although mutual trophic interactions between thymic microenvironment and developing thymocytes exist,33 this variation was not accompanied by obvious differences in major thymocyte subpopulations. Furthermore, the age-related decrease in this ratio was similar in Tgfb2+/-and wt mice. It is therefore unlikely that this phenotype would explain any differences in thymic involution. Finally, thymic involution overall proceeds more slowly in Tgfb2+/- mice. Our reciprocal transplantation experiments between wt and Tgfb2+/- mice indicate that increased expression of TGF-β2 within the hematopoietic system is sufficient to accelerate thymic involution in Tgfb2+/-mice. Because TGF-β2 can act on HSCs in a cell autonomous fashion, this finding may suggest that the accelerating effect of TGF-β2 on thymic involution is mediated by its action on HSCs or their direct progeny. Further support for this contention is provided by the fact that the biphasic TGF-β2 dose response on the proliferation of LSK cells in vitro is specific for LSK cells. In other cell types, including NIH 3T3 cells, PHA-stimulated T cells, a variety of leukemic and nonleukemic cell lines, and LSK cells immortalized with an Lhx2 expressing retroviral vector,35 TGF-β2, like TGF-β1 and TGF-β3, was an inhibitor of proliferation (J.C.L., S. Pal, and H.-W.S., unpublished data, December 2004). Furthermore, the particular biphasic dose response of TGF-β2 on the proliferation of LSK cells requires a serum factor. In serum-free conditions, the TGF-β2 dose response is entirely inhibitory and is not subject to quantitative genetic variation.28 It is therefore the LSK cell–specific, serum-dependent, positive regulatory effect of TGF-β2 that is subject to genetically determined variation and correlates with the rate of thymic involution. It cannot be excluded, however, that TGF-β2 has similar signaling characteristics in yet untested hematopoietic cell types, or that paracrine secretion of TGF-β2 by hematopoietic cells targets a nonhematopoietic cell that is critical for thymic involution. A final argument in favor of an effect of TGF-β2 on the early stage of hematopoietic differentiation is that ETP frequencies are higher in aged Tgfb2+/- mice. Thus, our findings lend support to, but do not prove, the controversial idea that functional characteristics of HSCs or their progeny may directly contribute to thymic involution.
If the role of TGF-β2 in thymic involution is mediated through its genetically determined, positive regulatory effect on the initial stages of hematopoiesis in vivo, how could TGF-β2 deficiency then decrease the rate of thymic involution? Tgfb2+/- HSCs cycle more slowly than wt HSCs.25 It is attractive to speculate that a lower level of cycling in the HSC compartment may delay the age-related loss of lymphoid potential,15-18 a process likely intrinsic to HSCs,34 and therefore decrease the rate of thymic involution. However, the absence of a statistically significant effect of the age of donor bone marrow cells on thymic involution in recipients suggests that, the genotype of repopulating HSC being equal, thymic involution is to a large extent determined by the aged environment. There are 2 ways these data can be reconciled with a role for TGF-β2 in thymic involution through its effect on the HSC compartment. One explanation is that in the reciprocal transplantation experiments, the reconstituting HSC are 14 months old in the aged recipients of young bone marrow, and 30 months old in the aged recipients of aged bone marrow. It is possible that any effect of aged HSCs may have been difficult to discern at that stage because the thymic involution curve levels off in aged mice. A second explanation is that not the age of HSCs, but other aspects of the function of HSCs, which do not change significantly with age but become biologically relevant only at a relatively old age, can affect the rate of thymic involution. Tgfb2+/- HSCs or ETPs may, for example, have a homing, growth, or differentiation advantage compared with wt cells in the microenvironment present in an aged thymus, but not in a young thymus.
The fraction of naive T cells was higher in aged Tgfb2+/- mice than in aged wt mice, indicating that the lower rate of thymic involution in Tgfb2+/- mice is biologically significant. It cannot be excluded that the difference in naive T cells' frequencies between aged Tgfb2+/- and wt mice, and among recipients of reciprocal transplants are caused by a direct effect of TGF-β2 on naive-memory ratios independent from the thymus. However, within each group of recipients in the reciprocal transplantation experiments, the correlation coefficient between thymic cellularity, which showed relatively large variation, and naive T-cell frequency was similar, independent of whether donor, recipient or both were wt or Tgfb2+/- (r = 0.789 for het→wt and wt→het, r = 0.655 for wt→wt, and r = 0.831 for het→het transplants). This finding argues against the contention that TGF-β2 directly affects naive-memory ratios. Hence, the frequency of naive T cells is very strongly associated with thymic cellularity and therefore likely a reflection of thymic function.
Wherever we observed variation in thymic involution in our study, correlating changes were found in the fraction of naive T cells. A higher frequency of naive CD4 cells and a reciprocally lower frequency of memory CD4 cells in the peripheral blood of aged mice have been shown to be associated with longer lifespan.36 In this context, it is interesting to note that we have previously demonstrated that QTLs regulating hematopoiesis, including those contributing to LSK cell frequency and responsiveness to TGF-β2, and QTLs contributing to genetic variation in lifespan are closely linked at multiple loci. These observations suggest that the HSC compartment may play a role in organismal aging.24 Although thymic involution in younger individuals may be developmentally regulated and is likely under evolutionary selection, further thymic involution in older individuals may be detrimental to health, and perhaps, to longevity.2,3,31,37 The aged immune system is characterized by impaired immune responses and a decreased pool of naive T cells, limiting the capacity of aged individuals to mount immune responses to neoantigens.2-4,31,36 Through peripheral expansion, memory cells fill the void in the T-cell pool caused by the decreased production of naive T cells. Senescent memory cells may contribute to the general state of low level inflammation that characterizes aging (“inflammaging”) in humans.31,37 Thus, our data raise the hypothesis that HSCs may affect lifespan through their effect on thymic involution and the concomitant depletion of naive T cells and expansion of memory T cells.
In conclusion, TGF-β2 accelerates thymic involution. Thymic involution is to a large extent determined by the aged environment, but TGF-β2 plays a major modulatory role that is subject to genetic variation and is possibly mediated through its regulatory effects on early hematopoiesis.
Prepublished online as Blood First Edition Paper, November 10, 2005; DOI 10.1182/blood-2005-04-1495.
Supported by National Institutes of Health grants RO1 AG16327 and R01 HL073760 (H.-W.S.).
R.K. performed studies on naive T cells, histology, flow cytometry and analyzed transplantations; J.C.L. set up transplantations and performed initial thymic involution studies in aged mice; and H.-W.S. designed the experiments and wrote the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal