Culturing mouse bone marrow in the presence of catalase dramatically alters hematopoiesis. Granulocyte output is initially increased 4- to 5-fold. This increase is transient and granulocyte production declines as immature (Sca-1+/LIN-) cells accumulate. One third of these immature cells have a phenotype (Sca-1+/c-Kit+) characteristic of hematopoietic stem cells. At 2 to 3 weeks there are greater than 200-fold more Sca-1+/c-Kit+/LIN- cells in treated cultures than in controls. This population contains functional stem cells with both short-term and long-term bone marrow repopulating activity. In addition to myeloid progenitors, this Sca-1+/LIN- population contains a large number of cells that express CD31 and CD34 and have an active Tie-2 promoter, indicating that they are in the endothelial lineage. After 3 to 4 weeks hematopoiesis in treated cultures wanes but if catalase is removed, hematopoiesis resumes. After 7 to 10 days the cultures are indistinguishable from untreated controls. Thus, protected from H2O2, hematopoietic progenitors multiply and become quiescent. This sequence resembles in vivo development in normal marrow. These results make it clear that peroxide-sensitive regulatory mechanisms play an important role in controlling hematopoiesis ex vivo and presumably in vivo as well. They also indicate that manipulation of the peroxide levels can be used to enhance the growth of hematopoietic stem cells in culture.
Introduction
Many of the events that occur within the bone marrow can be modeled in long-term bone marrow cultures (LTBMCs).1-6 These cultures are capable of producing hematopoietic stem cells (HSCs) as well as mature granulocytes, monocytes, immature erythrocytes, and megakaryocytes, and under appropriate conditions lymphoid lineage progenitors develop.7 Although the cultures replicate the differentiation of many hematopoietic lineages, they are relatively short-lived. The stem cell compartment of these cultures is rapidly depleted and attempts to achieve expansion of HSCs in culture have met with limited success.8,9 These cultures accumulate large numbers of granulocytes and monocytes that are capable of producing significant levels of reactive oxygen species (ROSs) including hydrogen peroxide and these ROSs may limit the life expectancy of the cultures.10
Historically ROSs have been of interest because of their ability to oxidize biologic macromolecules including proteins as well as DNA and lipids. These modifications can both produce necrosis and can provide a strong proapoptotic signal leading to cell death in the immediate vicinity of the ROS source.11,12 The toxicity of ROSs is modified by cellular enzymes that scavenge ROSs and by reactivity with a variety of antioxidants. The regulation of oxidative stress has been shown to be necessary for the maintenance of the capacity of HSCs to self-renew. Mice deficient in the ataxia telangiectasia mutated (Atm) gene, which contributes to the maintenance of genomic stability by activating a cell-cycle checkpoint in response to DNA damage, telomeric instability, or oxidative stress,13 have impaired hematopoiesis. The defect, which alters the capacity of the HSCs to self-renew but does not impair their capacity to differentiate, can be corrected by treatment with ROS scavengers.14 It has been suggested that ATM might be involved in oxidative defense against DNA damage through the induction of the major antioxidative systems including catalase.15
Several signaling pathways have been shown to be sensitive to the level of H2O2 in the environment. Recently it has become clear that some ROSs, including hydrogen peroxide, play a critical role in intracellular signaling. Many of the signaling effects are mediated through alterations in protein phosphorylation.16-18 H2O2 can modulate the function of protein tyrosine phosphatases. Protein tyrosine phosphatases (PTPases) contain a critical cysteine residue in their active site that is a potential target for redox regulation and which must be in the reduced state for full phosphatase activity.16,17,19,20 For example, H2O2 can specifically inhibit the PTPase activity of LAR (leukocyte antigen-related) and protein tyrosine phosphatase-1.21 Inhibition of PTPase through redox modulation may explain the broad spectrum of H2O2 on biologic activities.
In this report we demonstrate that the addition of catalase to the medium of LTBMCs significantly alters hematopoiesis in these cultures. The results are complex and change with time. The initial effect is to increase the production of granulocytes. As the cultures develop, maturation of hematopoietic progenitor cells (HPCs) is retarded and Sca-1+/LIN- cells accumulate. After approximately 3 weeks in culture this population has expanded by several hundred fold. It includes cells that express endothelial markers as well as cells that function as HSCs. These data clearly demonstrate that ROSs can play a regulatory role in stem cell renewal and differentiation in culture. Catalase-treated cultures also provide a remarkable source of early hematopoietic progenitors that may prove useful clinically.
Materials and methods
Primary stromal layer
The femurs and tibiae of a C57Bl/6 mouse are removed and the medullary cavities aseptically flushed with LTBMC medium using a 22-gauge needle and 6-mL syringe into three 25-cm2 tissue culture flasks. The flasks are incubated at 33°C, 5% CO2 in a humidified incubator. Half of the media in each flask is replaced with an equal amount of fresh media each week. At the end of the third week the nonadherent cells are removed and the culture trypsinized and replated in the same media at a 1:4 dilution. When the adherent layer reaches confluence the flasks are irradiated (450 cGy) to eliminate residual hematopoietic cells. In some experiments the monolayer was irradiated with 2Gy or was treated with mitomycin C (10 μg/mL for 1 hour).
Low-density, nonadherent bone marrow cells
Bone marrow from the femurs and tibiae of either B6.SJL-Ptprca Pep3b/BoyJ (Ly5.1) or FVB/N-TgN(Tie2/GFP)287Sato (Tie2/GFP) mice is collected in IMDM medium supplemented with 5% serum. A single-cell suspension is prepared by passing the suspension through a 27-gauge needle. This suspension is underlayed with an equal volume of ficoll hypaque layer (Histopaque-1077; density 1.077; Sigma, St Louis, MO) and centrifuged at 400g for 20 minutes at room temperature. Cells that accumulate at the interface are collected. Cells are counted using a Coulter counter Model Zf (Hialeah, FL). Low-density cells are plated at a density of 5 × 106 cells/mL in IMDM with 5% serum and incubated at 37°C in 5% CO2 for 3 hours. Nonadherent cells are collected by removing the medium, washing the plate twice with prewarmed IMDM media, and pooling the washes with the original medium.
Long-term bone marrow culture
Bone marrow cells (total bone marrow or low-density, nonadherent cells) are plated on preformed, irradiated stromal layers in LTBMC media (Stem Cell Technologies, Vancouver, BC, Canada; M5300). An appropriate amount of catalase is added and the cultures are incubated at 33°C with 5% CO2 in a humidified incubator. Additional catalase is added on alternate days to maintain the level of catalase activity in the cultures. The cultures are fed once a week with fresh LTBMC medium after demi-depletion. At the time of feeding, nonadherent cells are collected by centrifugation and resuspended in IMDM medium with 5% serum. All of the experiments described here were repeated at least 3 times. In order to determine if the cultures would recover from catalase treatment, the following experiment was performed. After 4 weeks of culture (± catalase) the medium (with the nonadherent cells) was removed. The adherent layer was washed with fresh medium and the nonadherent cells were pelleted (600 g × 10 minutes) and resuspended in fresh medium without catalase. These cells, in fresh catalase-free medium, were returned to flasks in which they had grown. Hematopoiesis was assessed for the next 2 weeks. This experiment was also repeated 3 times.
Colony-forming units in culture (CFU-Cs)
Twenty thousand nonadherent or 10 000 nonadherent and adherent cells from control culture flasks and catalase-treated flasks or 5000 low-density mouse bone marrow cells are plated in methyl cellulose medium (M3434; Stem Cell Technologies) in 6-well tissue culture plates. The plates are placed in a sealed plastic bag along with an open Petri dish containing sterile water and incubated at 37°C, 5% CO2 for 5 to 17 days and the number of colony-forming units are determined.
Immunofluorescence
Cultured cells (105) or mouse bone marrow cells in a final volume of 100 μL are incubated with various antibodies (CD45-FITC, CD31-PE, c-Kit PE [clones YW 62.3, 390, and 2B8 purchased from Caltag Laboratories, Burlingame, CA]; CD45.1-biotin, Sca1-APC, CD16/32-PEcy7, CD34-biotin, IL7Rα-biotin procured from eBioscience [San Diego, CA; clones A20, D7, 93, RAM34, A7R34]; Sca1 PE and CD8-FITC purchased from BD-Biosciences [San Diego, CA; clones D7 and 53]) and a cocktail of antibodies against lineage markers labeled with FITC. Lineage-specific antibodies GR1-PE and GR1-Fitc, CD4, B220 are purchased from Caltag Laboratories (clones RB6-8C5, CT-CD4, RA3-6B2); Cd11b, Ter-119, CD3 are from eBioscience (clones M1/70, TER-119, 145-2C11). All staining is performed at limiting dilution of antibodies for 30 minutes at room temperature. Cells stained with biotinylated antibodies are washed once with PBS and labeled with either avidin-PE-Cy7, avidin-PE-Cy5, or streptavidin-APC. The cells are washed again with PBS and fixed by adding 0.2 mL of 0.2% paraformaldehyde, pH 7.5. In most experiments the cells are analyzed using a FACS Vantage flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) but the 5-color analysis is performed using a MoFlo Cytometer and Sorter (Cytomation, Fort Collins, CO).
Mouse low-density bone marrow cells or culture-treated cells are sorted in a MoFlo Sorter after staining with Sca1-PE or lineage-FITC cocktail containing Gr1, CD11b, B220, CD4, CD3, CD8, and TER119 as described. The stained cells are not fixed and are suspended in PBS containing 5% serum. The sorted cells are collected in PBS+serum.
Repopulation of lethally irradiated mice with culture-treated cells
C57Bl/6 mice (Ly5.2; 5-8 mice per group) are irradiated with 1100 cGy (550 cGy × 2, 3 hours apart) and then injected intravenously with SJL/pep3b (Ly5.1) BM cells from either fresh marrow or adherent and nonadherent cells from control and catalase-treated cultures. Mice in all the test groups also received 30 000 low-density (LD) syngeneic BM cells. After 3, 6, and 10 weeks, the mice are bled and the peripheral blood leukocytes (PBLs) analyzed for the presence of donor-derived lymphocytes (CD3 and B220) and myeloid cells (Gr-1 and Mac-1).
Results
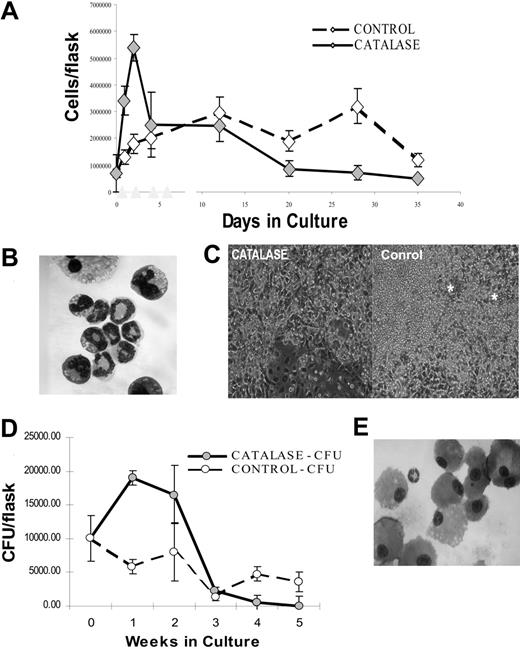
The addition of bovine catalase to murine LTBMCs produces dramatic alterations in hematopoiesis. Initially, there is a large (5-10 fold) increase in the number of nonadherent hematopoietic cells in the culture (Figure 1A). Greater than 90% of these cells are myelocytes, metamyelocytes, and granulocytes (Figure 1B). These are the small refractile cells seen in the photomicrograph (Figure 1C). This increase is relatively short-lived and by the end of 3 to 4 weeks catalase-treated cultures contain only approximately one fifth as many hematopoietic cells as do controls (Figure 1A). Although mature myeloid cells decline after the first week, catalase-containing cultures contain increased numbers of myeloid progenitors (CFU-Cs) for several weeks (Figure 1D). At the maximum, the catalase cultures contain up to 10 times the number of granulocytes and approximately 5 times the number of clonal progenitors. Because the increase in cell number is short-lived and ends at a time that progenitor number is still high, the net effect is to produce a significant enrichment of CFUs among the cells removed from the cultures.
We are confident that these effects are caused by catalase and not some impurity in the preparation. Neither denatured catalase (heated to 90°C) nor superoxide dismutase, another ROS scavenger, has any effect on these cultures (data not shown). The addition of the structurally unrelated but functionally similar catalase from Aspergillus produces a 2-fold increase in clonal progenitors in LTBMCs (Table 1). The effects of bovine catalase are dose dependent up to 100 μg/mL (Table 1). Because bovine catalase is not stable in these cultures, optimal results were obtained by adding catalase every other day. With time, hematopoiesis diminishes in the catalase-treated cultures. At 3 to 4 weeks the only hematopoietic cells visible using phase microscopy are large monocytic cells (Figure 1C, left panel). In contrast the control cultures are actively hematopoietic with many granulocytes (small phase bright cells) and numerous “cobblestone” areas (phase dense cells growing under the stromal layer, marked with *).
Dose response of the effect of catalase on mouse LTBMCs
Catalase . | Concentration, μg/mL . | Times replenished/week . | Cells/flask, × 106* . | CFUs per 1000 cells . | CFUs/flask† . |
|---|---|---|---|---|---|
| None | 0 | 3 | 4.7 | 0.35 | 1 650 ± 366 |
| Bovine | 50 | 3 | 1.2 | 5.61 | 6 390 ± 515 |
| Bovine | 100 | 3 | 1.0 | 12.11 | 12 110 ± 663 |
| Bovine | 200 | 3 | 1.4 | 6.11 | 8 870 ± 1 610 |
| None | 0 | 1 | 1.3 | 0.29 | 377 ± 103 |
| Aspergillus | 10 | 1 | 0.35 | 1.51 | 528 ± 97 |
| Aspergillus | 30 | 1 | 0.40 | 1.95 | 780 ± 128 |
| Aspergillus | 60 | 1 | 0.25 | 2.45 | 604 ± 320 |
Catalase . | Concentration, μg/mL . | Times replenished/week . | Cells/flask, × 106* . | CFUs per 1000 cells . | CFUs/flask† . |
|---|---|---|---|---|---|
| None | 0 | 3 | 4.7 | 0.35 | 1 650 ± 366 |
| Bovine | 50 | 3 | 1.2 | 5.61 | 6 390 ± 515 |
| Bovine | 100 | 3 | 1.0 | 12.11 | 12 110 ± 663 |
| Bovine | 200 | 3 | 1.4 | 6.11 | 8 870 ± 1 610 |
| None | 0 | 1 | 1.3 | 0.29 | 377 ± 103 |
| Aspergillus | 10 | 1 | 0.35 | 1.51 | 528 ± 97 |
| Aspergillus | 30 | 1 | 0.40 | 1.95 | 780 ± 128 |
| Aspergillus | 60 | 1 | 0.25 | 2.45 | 604 ± 320 |
Total number of nonadherent cells in the cultures and colony-forming units per culture measured at week 2 (for bovine catalase) and week 3 (Aspergillus catalase)
CFUs per cells plated in methylcellulose × cells recovered per flask ± SEM
Effect of catalase treatment on LTBMCs. (A) Nonadherent cells recovered in control and catalase-treated (100 μg/mL) cultures (mean per culture ± SD). Low-density, nonadherent cells were cultured as described in “Materials and methods.” Each point is the mean of 3 to 5 replicate cultures. The results illustrated are representative of those found in greater than 10 repetitions of this experiment. (B) Wright-Giemsa-stained cytospin of the nonadherent cells removed from the culture after 1 week. There is a preponderance of granulocytes at varying stages of maturation. Original magnification, × 600; 60×/1.40 numeric aperture (NA) objective on a Leitz ortholux microscope (Leica Microsystems, Bannockburn, IL) and a Nikon D100 digital camera (Melville, NY). (C) Photomicrograph of phase contrast images showing differences in control and catalase-treated (100 μg/mL) cultures after 24 days of culture. Original magnification, × 100; 10 ×/0.25 NA objective. * indicates the cobblestone areas. The images were obtained using a Nikon D100 digital camera mounted on a Nikon inverted microscope. (D) CFU-Cs in the nonadherent cells recovered from control and catalase-treated (100 μg/mL) cultures. (E) Wright-Giemsa-stained cytospin of the nonadherent cells removed from the culture illustrated in panels A and D after 3 weeks. Almost all of the cells appear to be mature monocytes and/or macrophages. Original magnification, × 600 as described. (A, D) Error bars indicate SD of the mean.
Effect of catalase treatment on LTBMCs. (A) Nonadherent cells recovered in control and catalase-treated (100 μg/mL) cultures (mean per culture ± SD). Low-density, nonadherent cells were cultured as described in “Materials and methods.” Each point is the mean of 3 to 5 replicate cultures. The results illustrated are representative of those found in greater than 10 repetitions of this experiment. (B) Wright-Giemsa-stained cytospin of the nonadherent cells removed from the culture after 1 week. There is a preponderance of granulocytes at varying stages of maturation. Original magnification, × 600; 60×/1.40 numeric aperture (NA) objective on a Leitz ortholux microscope (Leica Microsystems, Bannockburn, IL) and a Nikon D100 digital camera (Melville, NY). (C) Photomicrograph of phase contrast images showing differences in control and catalase-treated (100 μg/mL) cultures after 24 days of culture. Original magnification, × 100; 10 ×/0.25 NA objective. * indicates the cobblestone areas. The images were obtained using a Nikon D100 digital camera mounted on a Nikon inverted microscope. (D) CFU-Cs in the nonadherent cells recovered from control and catalase-treated (100 μg/mL) cultures. (E) Wright-Giemsa-stained cytospin of the nonadherent cells removed from the culture illustrated in panels A and D after 3 weeks. Almost all of the cells appear to be mature monocytes and/or macrophages. Original magnification, × 600 as described. (A, D) Error bars indicate SD of the mean.
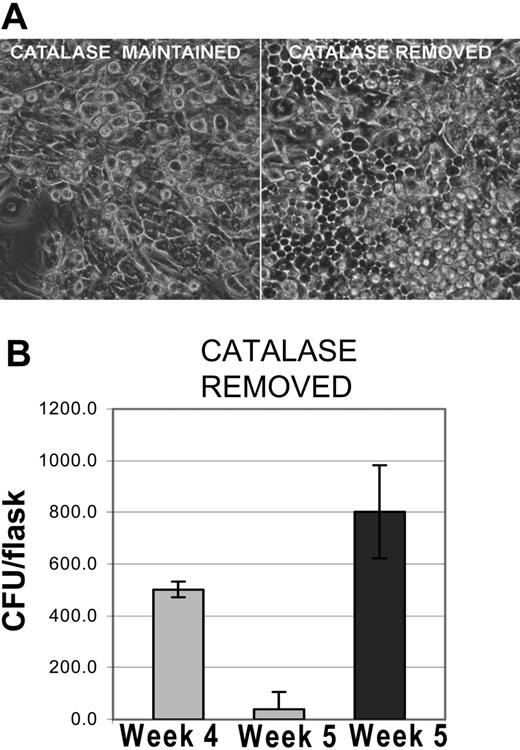
Restoration of hematopoiesis in cultures after the removal of catalase. (A) Recovery of hematopoiesis after the removal of catalase. Photomicrograph of phase contrast images showing differences between cultures maintained in catalase for 5 weeks and cultures from which catalase was removed after 4 weeks. Original magnification, × 100 as described in Figure 1C. (B) Recovery of clonal progenitors in LTBMCs after the removal of catalase after week 4. The results shown are representative of 3 similar experiments and the data shown are the mean and SD of 3 to 5 replicates.
Restoration of hematopoiesis in cultures after the removal of catalase. (A) Recovery of hematopoiesis after the removal of catalase. Photomicrograph of phase contrast images showing differences between cultures maintained in catalase for 5 weeks and cultures from which catalase was removed after 4 weeks. Original magnification, × 100 as described in Figure 1C. (B) Recovery of clonal progenitors in LTBMCs after the removal of catalase after week 4. The results shown are representative of 3 similar experiments and the data shown are the mean and SD of 3 to 5 replicates.
After 4 to 5 weeks the catalase-treated cultures become quiescent; mitosis stops and only monocytes remain as identifiable hematopoietic cells (Figure 1E and 2A left panel). Despite this appearance hematopoietic progenitors are still present. When catalase is removed from the medium, hematopoiesis returns promptly. Within 72 hours cobblestone areas are readily observable (Figure 2A, right panel) and clonal progenitors reappear (Figure 2B). Within 1 week flow cytometric analyses of the cultures are indistinguishable from those obtained from cultures of the same age that had not been exposed to catalase (data not shown). In most of the experiments the stromal cells were irradiated with 450 cGy, which eliminates any residual hematopoietic cells in the stroma, but all of these effects also occurred in cultures in which the stromal cells received 2 Gy or were treated with mitomycin C (data not shown). Since these treatments inhibit new mRNA by the cells of the preformed stroma, catalase-dependent effects must be due to changes in the progeny of the low-density bone marrow cells.
It has been known for some time that in long-term cultures of mouse bone marrow, more hematopoietic progenitors are found associated with the stromal layer than are found free in the medium. If catalase treatment were only to alter the distribution of the progenitors, more CFU-Cs would be found in the nonadherent fraction without any overall increase in progenitors. The adherent layer would be depleted of progenitors. To test this possibility, we harvested the progenitor population associated with the adherent layer by trypsinizing the layer (after the removal of the nonadherent cells). A single suspension was prepared, fibroblasts removed by allowing them to reattach to plates, and the newly released and now nonadherent cells assayed. The results are shown in Table 2. Progenitors in both the nonadherent and the adherent compartments were increased by catalase treatment. The changes induced by catalase were of similar magnitude in both locations and the increased number of progenitors found in the nonadherent fraction cannot be the result of redistribution of progenitors from the adherent layer.
Effect of catalase on the distribution of progenitors in LTBMCs
. | Adherence . | Cell count/culture, × 106 . | CFUs/100 000 cells . | CFUs/culture . |
|---|---|---|---|---|
| Control | No | 2.6 | 32 | 832 |
| Control | No | 2.4 | 20 | 480 |
| Catalase | No | 2.3 | 180 | 4 140 |
| Catalase | No | 2.0 | 370 | 7 400 |
| Control | Yes | 2.5 | 62 | 1 550 |
| Control | Yes | 4.1 | 32 | 1 312 |
| Catalase | Yes | 3.1 | 250 | 7 750 |
| Catalase | Yes | 4.0 | 542 | 21 680 |
. | Adherence . | Cell count/culture, × 106 . | CFUs/100 000 cells . | CFUs/culture . |
|---|---|---|---|---|
| Control | No | 2.6 | 32 | 832 |
| Control | No | 2.4 | 20 | 480 |
| Catalase | No | 2.3 | 180 | 4 140 |
| Catalase | No | 2.0 | 370 | 7 400 |
| Control | Yes | 2.5 | 62 | 1 550 |
| Control | Yes | 4.1 | 32 | 1 312 |
| Catalase | Yes | 3.1 | 250 | 7 750 |
| Catalase | Yes | 4.0 | 542 | 21 680 |
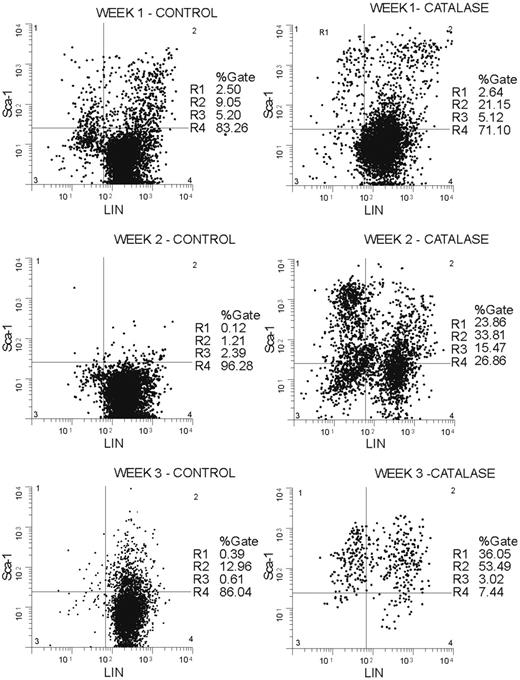
The increase in progenitors Sca-1+, Gr-1-, Mac-1-, Ter 119-, CD3-, and CD4- (lineage negative; LIN-) cells accumulate in catalase-treated LTBMCs (Figure 3). By the third week of culture greater than 90% of the nonadherent cells were Sca-1+ and a significant proportion of these (36% of 90%) were LIN-. In terms of absolute numbers, the Sca-1+/LIN- population increases 80-fold from approximately 3500 cells (0.5% of 7 × 105 low-density BMCs used to initiate the cultures) to approximately 240 000 (24% of 1.05 × 106) cells after 2 weeks in culture and reaches a maximum of approximately 280 000 (36% of 780 000) cells at week 3. If the losses caused by the removal of half of the nonadherent cells at each weekly feeding are taken into account, the absolute increase in Sca-1+/LIN- cells is greater than 500-fold.
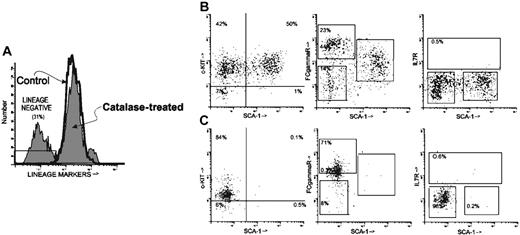
The Sca-1+/LIN- phenotype is characteristic of HSCs and early myeloid progenitors.22-24 It is a heterogeneous population and can be subdivided on the basis of the expression of c-Kit, FcγR (CD16/CD32), and IL7Rα. Thus, in another experiment, after 18 days in culture 30.5% of the cells removed from catalase-treated cultures were LIN- (Figure 4A; Table 3). After catalase treatment 50% of the LIN- cells were Sca-1+ and virtually all of these Sca-1+/LIN- cells are c-Kit+ (49.6% Sca-1+ c-Kit+ of 50.5% Sca-1+ cells). Approximately 15% of the cells recovered from LTBMCs grown in the presence of catalase have the phenotype described for HSCs. This Sca-1+/LIN-/c-Kit+ population also expresses intermediate amounts of FcγR (Figure 4B, middle) but does not express IL7Rα (Figure 4B, right). In addition to cells with a stem cell phenotype these cultures contain many cells (∼23%) with the phenotype ascribed to a “clonogenic common myeloid progenitor.”23 These cells, which are Sca-1-/LIN- and FcγR bright (Figure 4B, middle), are also predominantly c-Kit+ (Figure 4B, left). Cells with the phenotype of the “common lymphoid progenitor” (IL7Rα+/Sca-1low/c-Kitlow)24 were not detected (Figure 4B, right, C, right). Cells with a stem cell phenotype (Sca-1+/LIN-/c-Kit+) were approximately 200 to 500 times more plentiful in the catalase-treated cultures than in the control (Figure 4B, right, C, right; Table 3).
Recovery of Sca-1+ and c-Kit+ cells from catalase-treated and control cultures
. | Control . | . | . | Catalase . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. of cells . | % of total . | % of LIN- . | No. of cells . | % of total . | % of LIN- . | ||||
| Total | 5 600 000 | 100.0 | — | 260 000 | 100.0 | — | ||||
| Lineage negative | 140 000 | 2.5 | 100.0 | 79 248 | 30.5 | 100.0 | ||||
| Sca-1+ | 840 | 0.0 | 0.6 | 40 020 | 15.5 | 50.5 | ||||
| c-Kit+ | 117 320 | 2.1 | 83.8 | 72 670 | 28.0 | 91.7 | ||||
| Sca-1+/c-Kit+ | 211 | 0.0 | 0.15 | 39 307 | 15.1 | 49.6 | ||||
. | Control . | . | . | Catalase . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | No. of cells . | % of total . | % of LIN- . | No. of cells . | % of total . | % of LIN- . | ||||
| Total | 5 600 000 | 100.0 | — | 260 000 | 100.0 | — | ||||
| Lineage negative | 140 000 | 2.5 | 100.0 | 79 248 | 30.5 | 100.0 | ||||
| Sca-1+ | 840 | 0.0 | 0.6 | 40 020 | 15.5 | 50.5 | ||||
| c-Kit+ | 117 320 | 2.1 | 83.8 | 72 670 | 28.0 | 91.7 | ||||
| Sca-1+/c-Kit+ | 211 | 0.0 | 0.15 | 39 307 | 15.1 | 49.6 | ||||
The Sca-1+/c-Kit+ (catalase)/Sca-1+/c-Kit+ (control) ratio = 186.3.
— indicates not applicable.
Sca-1+/LIN- cells accumulate in catalase-treated LTBMCs. Nonadherent cells removed from culture each week were stained with antibodies against murine Sca-1 labeled with phycoerythrin (PE) and a lineage marker cocktail (Gr-1, Mac-1, and Ter-119) labeled with FITC. Plot showing percentage of Sca-1+ and lineage-negative cells at weeks 1, 2, and 3 in catalase-treated cultures (right panel) versus control cultures (left panel). The histograms shown are representative of those obtained repeatedly over a 2-year period.
Sca-1+/LIN- cells accumulate in catalase-treated LTBMCs. Nonadherent cells removed from culture each week were stained with antibodies against murine Sca-1 labeled with phycoerythrin (PE) and a lineage marker cocktail (Gr-1, Mac-1, and Ter-119) labeled with FITC. Plot showing percentage of Sca-1+ and lineage-negative cells at weeks 1, 2, and 3 in catalase-treated cultures (right panel) versus control cultures (left panel). The histograms shown are representative of those obtained repeatedly over a 2-year period.
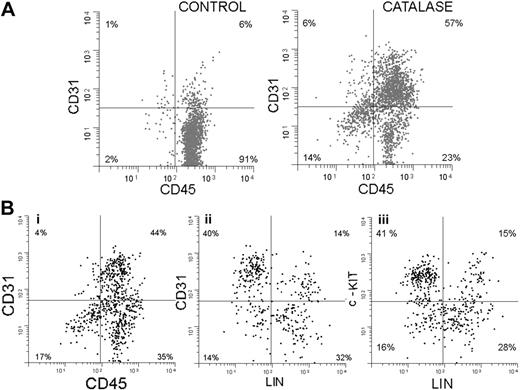
In addition to the increase in Sca-1+ cells, the catalase-treated cultures show a significant increase in cells expressing CD31. This is shown in Figure 5A. Sixty-three percent of the cells are CD31+ compared with only 7% in the control, and 90% (57/63) express Sca-1. Since after 3 weeks in culture, 90% of the nonadherent cells express Sca-1 and almost half of these express no lineage markers (Gr-1, Mac-1, Ter 119, CD3, or CD4), we have focused on this Sca-1+/LIN- population.
In another experiment, using 3-color immunofluorescence, we further explored the phenotype of these Sca-1+ cells and the results are shown in Figure 5B. Only results from catalase-treated cultures are shown and the results have been electronically gated on Sca-1+ cells. Seventy-nine percent of the Sca-1+ cells in the culture are CD45+ (Figure 5Bi). Forty-four percent express both CD45 and CD31, suggesting that they are at a very early stage of development. The results in Figure 5Bii show that most of the Sca-1+ CD31+ cells (which are also CD45+ as shown in Figure 5Bi) are lineage negative, which is consistent with their immature status. Figure 5Biii demonstrates that most of the Sca-1+, LIN- cells are c-Kit+. The Sca-1+, CD45+, CD31+ phenotype is consistent with an even more immature cell, the hemangioblast. This cell is thought to be able to give rise to both the hematopoietic and vascular endothelial lineages.25-27
Phenotype of lineage-negative cells from catalase-treated LTBMCs. Nonadherent cells removed from culture at week 2 were stained with an antilineage cocktail (FITC) and anti-c-Kit (PE), Sca-1 (APC), FcγR (PE-Cy7), and IL7Rα (biotin/streptavidin-tricolor). Representative histograms are shown but similar results have been obtained repeatedly. (A) Histogram showing the staining with the lineage cocktail in the control and catalase-treated cultures. The region labeled LIN- was then used as a gate to analyze the distribution of the other markers. (B) LIN- cells from catalase-treated cultures were further analyzed for their expression of Sca-1 (x-axis in all 2-dimensional histograms) and for the expression of c-Kit, FcγR, and IL7Rα. (C) LIN- cells from control cultures analyzed as in panel B.
Phenotype of lineage-negative cells from catalase-treated LTBMCs. Nonadherent cells removed from culture at week 2 were stained with an antilineage cocktail (FITC) and anti-c-Kit (PE), Sca-1 (APC), FcγR (PE-Cy7), and IL7Rα (biotin/streptavidin-tricolor). Representative histograms are shown but similar results have been obtained repeatedly. (A) Histogram showing the staining with the lineage cocktail in the control and catalase-treated cultures. The region labeled LIN- was then used as a gate to analyze the distribution of the other markers. (B) LIN- cells from catalase-treated cultures were further analyzed for their expression of Sca-1 (x-axis in all 2-dimensional histograms) and for the expression of c-Kit, FcγR, and IL7Rα. (C) LIN- cells from control cultures analyzed as in panel B.
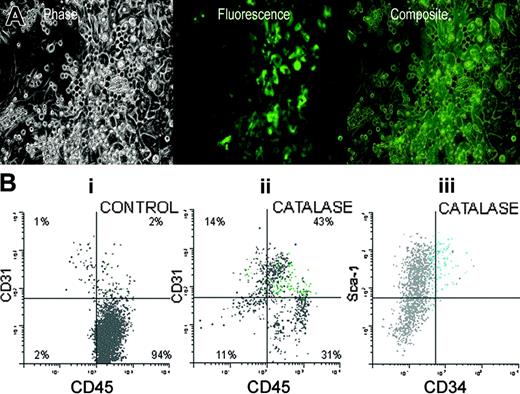
We also found that many of the catalase-cultured cells express GFP under the control of the Tie-2 promoter. Tie-2 signaling pathways are essential for normal vascular development in the embryo and are important in stabilization of mature blood vessels in the adult.28-30 When fused to the murine Tie-2 promoter, GFP expression is activated in all endothelial lineage cells. Tie-2-driven GFP expression is also seen in a small number of very immature hematopoietic precursors.31 In these mice, GFP expression in a tissue should correlate with the presence of endothelial cells or their precursors. We made use of this by using bone marrow (BM) cells from transgenic mice that express the green fluorescent protein (GFP) under the control of the Tie-2 promoter to initiate LTBMCs. Figure 6A is a phase-fluorescence composite of a 3-week-old culture and shows that both adherent cells and phase dense cells (presumably nonadherent cells) express GFP. Note that the cobblestone areas that are composed primarily of developing myeloid cells do not express GFP and that Tie-2/GFP expression is seen in both flattened adherent cells and in round cells that do not morphologically resemble endothelial cells.
Figure 6B shows a flow cytometric analysis of nonadherent cells produced in these cultures. Catalase treatment results in the accumulation of CD31+, CD45+ cells. Forty-three percent of the cells in the catalase-treated culture expressed both CD45 and CD31 (Figure 6Bii) compared with only 2% in the control cultures (Figure 6Bi). Many of these double-positive cells also expressed Tie-2-driven GFP (Figure 6Bii-iii, green dots). Eighty-seven percent of the total of Tie-2/GFP-positive cells fell in a quadrant used to define CD45+/CD31+ cells. The Tie-2/GFP+ cells also expressed CD34 (Figure 6Biii).
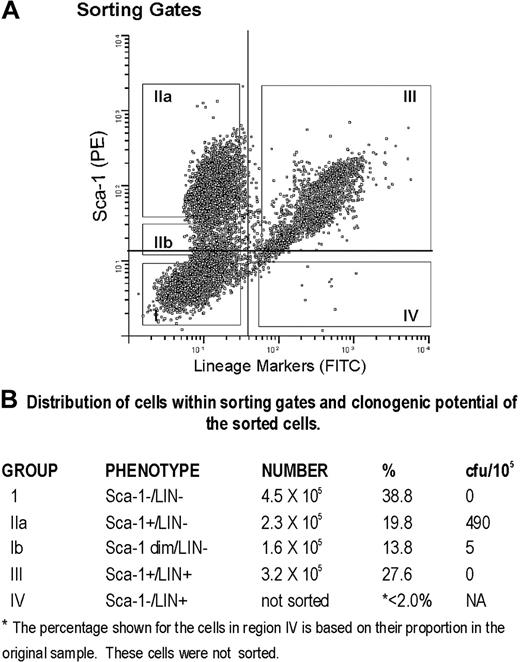
LTBMCs grown in the presence of catalase not only contain cells with the phenotype of early members of the hematopoietic lineage but also are enriched for functional precursors. Cells recovered from the catalase-containing cultures were stained with Sca-1 and a cocktail of lineage markers and then sorted into 4 fractions as shown in Figure 7. The Sca-1+/LIN- population (Figure 7, fraction IIa) contains almost all of the small number of CFU-Cs present in 3-week cultures (Figure 7B). The data shown are based on the number of cells recovered after sorting.
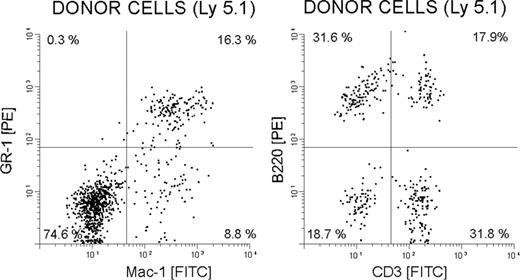
Catalase-cultured and control cells were also tested for their ability to restore hematopoiesis in lethally irradiated mice. In these experiments various numbers of cultured Ly5.1 low-density BM cells (± catalase for 20 days) were injected into the irradiated Ly5.2 mice along with 25 000 freshly isolated Ly5.2 BM cells (5-8 animals/group). The peripheral blood of the recipients was sampled thereafter. The results shown in Table 4 and Figure 8 demonstrate that the cells cultured in the presence of catalase are better able to provide hematopoietic support than cells in the controls and that the catalase-cultured cells are capable of multi-lineage repopulation. Ly5.1 granulocytes (Gr-1+ cells), monocytes (Mac-1+ cells), T cells (CD3+), and B cells (B220+) were all present in the Ly5.2 mice that received a transplant (Figure 8).
Proportion of CD45.1 (donor-derived) cells in peripheral blood
. | Percentage of CD45.1 donor cells, mean ± SEM . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Group and no. of cells injected* . | 4 weeks after transplantation . | 8 weeks after transplantation . | 12 weeks after transplantation . | 30 weeks after transplantation . | |||
| LTBMCs, cultured 3 weeks without catalase | |||||||
| 50 000 | 2.8 ± 0.3 | 6.4 ± 0.6 | 7.0 ± 1.1 | 5.4 ± 0.6 | |||
| 20 000 | 2.8 ± 0.7 | 4.5 ± 0.4 | 4.9 ± 0.7 | 4.8 ± 0.8 | |||
| 5 000 | 1.8 ± 0.8 | 1.2 ± 0.9 | 2.1 ± 0.2 | ND | |||
| LTBMCs, cultured 3 weeks with catalase | |||||||
| 50 000 | 7.1 ± 1.8‡ | 11.3 ± 1.6‡ | 22.6 ± 6.6‡§ | 10.4 ± 1.2‡ | |||
| 20 000 | 5.4 ± 1.0 | 12.9 ± 2.2‡ | 7.1 ± 1.4 | 8.2 ± 0.8‡ | |||
| 5 000 | 2.7 ± 0.5 | 3.1 ± 0.9 | 5.3 ± 0.3‡ | ND | |||
| Sorted Sca-1+/LIN- from catalase-treated culture† | |||||||
| 20 000 | 15.3 ± 4.9 | 23.0 ± 8.0 | 23.3 ± 4.6 | ND | |||
. | Percentage of CD45.1 donor cells, mean ± SEM . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Group and no. of cells injected* . | 4 weeks after transplantation . | 8 weeks after transplantation . | 12 weeks after transplantation . | 30 weeks after transplantation . | |||
| LTBMCs, cultured 3 weeks without catalase | |||||||
| 50 000 | 2.8 ± 0.3 | 6.4 ± 0.6 | 7.0 ± 1.1 | 5.4 ± 0.6 | |||
| 20 000 | 2.8 ± 0.7 | 4.5 ± 0.4 | 4.9 ± 0.7 | 4.8 ± 0.8 | |||
| 5 000 | 1.8 ± 0.8 | 1.2 ± 0.9 | 2.1 ± 0.2 | ND | |||
| LTBMCs, cultured 3 weeks with catalase | |||||||
| 50 000 | 7.1 ± 1.8‡ | 11.3 ± 1.6‡ | 22.6 ± 6.6‡§ | 10.4 ± 1.2‡ | |||
| 20 000 | 5.4 ± 1.0 | 12.9 ± 2.2‡ | 7.1 ± 1.4 | 8.2 ± 0.8‡ | |||
| 5 000 | 2.7 ± 0.5 | 3.1 ± 0.9 | 5.3 ± 0.3‡ | ND | |||
| Sorted Sca-1+/LIN- from catalase-treated culture† | |||||||
| 20 000 | 15.3 ± 4.9 | 23.0 ± 8.0 | 23.3 ± 4.6 | ND | |||
ND indicates not done.
Each group contained 9 or 10 mice, and each sample was analyzed in duplicate. Each mouse also received 25 000 fresh syngeneic (CD45.2) bone marrow cells to assure survival after irradiation
For sorted Sca-1+/LIN- cells from catalase-treated culture, percentage of CD45.1 donor cells was measured at 3, 7, and 10 weeks after transplantation
Value differs from the catalase-free control culture, with P values less than .05
This value includes the results from a single mouse that had more than 70% donor cells. If that outlying animal is eliminated, the resultant value would be 15.5% ± 4.1%
Endothelial markers expressed by lineage-negative cells from catalase-treated LTBMCs. (A) Two-dimensional histograms (cytograms) of CD31 (y-axis) plotted against CD45 (x-axis) after 3 weeks of culture with and without catalase. Sca-1+ cells are shown in black and Sca-1- cells are shown in gray. (B) Two-dimensional histograms (cytograms) showing the expression of (i) CD31 versus CD45, (ii) CD31 versus LN (lineage negative), and (iii) c-Kit versus LN by nonadherent cells collected after 3 weeks of culture in the presence of catalase.
Endothelial markers expressed by lineage-negative cells from catalase-treated LTBMCs. (A) Two-dimensional histograms (cytograms) of CD31 (y-axis) plotted against CD45 (x-axis) after 3 weeks of culture with and without catalase. Sca-1+ cells are shown in black and Sca-1- cells are shown in gray. (B) Two-dimensional histograms (cytograms) showing the expression of (i) CD31 versus CD45, (ii) CD31 versus LN (lineage negative), and (iii) c-Kit versus LN by nonadherent cells collected after 3 weeks of culture in the presence of catalase.
Table 4 shows the repopulation obtained in a series of experiments using cells that had been cultured with or without catalase for 3 weeks to reconstitute lethally irradiated mice. Groups of 8 mice were irradiated and reconstituted with 5000, 20 000, or 50 000 cells. The extent of repopulation was dose dependent and at all time points and at cell doses, the cells obtained from catalase-treated cultures had greater repopulating activity than the controls. When 50 000 catalase-treated cells were transferred, a statistically significant increase in repopulation of 3.1-, 2.5-, and 1.9-fold was noted at 3 to 4 weeks, 7 to 8 weeks, and 30 weeks, respectively. When 20 000 catalase-treated cells were transferred, a similar significant increase in repopulation was noted at 7 to 8 weeks (4.6-fold), 10 to 12 weeks (2.3-fold), and 30 weeks (1.8-fold). Since these differences were still apparent when the animals were killed 30 weeks after transplantation, we chose to kill these mice and determine if the cultured cells were capable of reconstituting irradiated mice after a second transfer. As is clear in Figure 9, cells from the animals reconstituted with catalase-cultured cells contained up to 6.7 times more culture-derived cells than did animals reconstituted with control cells.
Tie-2/GFP expression in catalase-treated LTBMCs. (A) Phase and fluorescent photomicrographs of LTBMCs were initiated on irradiated C57Bl/6 stroma, with low-density nonadherent Tie-2/GFP bone marrow cells and cultured for 3 weeks in the presence of catalase. Original magnification, × 200; digital images overlaid using Photoshop (Adobe, San Jose, CA). (B) In each of the figures cells that express the endogenous fluorescence of GFP have been shown as green dots. GFP-negative cells are gray.
Tie-2/GFP expression in catalase-treated LTBMCs. (A) Phase and fluorescent photomicrographs of LTBMCs were initiated on irradiated C57Bl/6 stroma, with low-density nonadherent Tie-2/GFP bone marrow cells and cultured for 3 weeks in the presence of catalase. Original magnification, × 200; digital images overlaid using Photoshop (Adobe, San Jose, CA). (B) In each of the figures cells that express the endogenous fluorescence of GFP have been shown as green dots. GFP-negative cells are gray.
An additional group of mice received transplants with catalase-cultured cells that had been sorted on the basis of Sca-1 and lineage marker expression. Sorted Sca-1+/LIN- cells (20 000) obtained from catalase-treated cultures produced extensive repopulation (15.3%-23.3% over the 3- to 12-week period), which exceeded the repopulation found in mice that had received an equivalent number of unsorted cells (data not shown) and was greater than that found in mice that had received 50 000 unsorted cells. Sca-1+/LIN- cells are so infrequent in the control culture that it was impossible to isolate sufficient cells to include in this control. The results indicate the repopulating cells obtained from catalase-treated cultures are better able to reconstitute the hematopoietic system of irradiated mice. In the animals reconstituted with the sorted cells, the differences between the control and catalase-treated cultures tend to increase with time, suggesting that the differences between the 2 groups are due to better preservation of long-term repopulating cells. The results of the sorting experiment show that repopulating cells present in the catalase-treated cultures have a phenotype that is similar to that of repopulating cells in uncultured BM cells.
Sorting of Sca-1+/LIN- cells from catalase-treated LTBMCs. Sorting gates used to define populations of cultured cells expressing Sca-1 and lineage markers.
Sorting of Sca-1+/LIN- cells from catalase-treated LTBMCs. Sorting gates used to define populations of cultured cells expressing Sca-1 and lineage markers.
Discussion
Catalase profoundly alters hematopoiesis in long-term BMCs. Among the early effects of catalase treatment is a dramatic increase in the number of cells expressing Sca-1. The increase is detectable within 48 hours and by the end of 3 weeks the vast majority (65%-90%) of the hematopoietic cells express Sca-1. Later the catalase-treated cultures accumulate a large number of cells that do not express antigens characteristic of the mature myeloid or lymphoid lineages. These Sca-1+/LIN- cells have the phenotype of primitive hematopoietic stem cells (PHSCs).32 Although all mouse PHSCs appear to have the Sca-1+/LIN- phenotype, all cells with this phenotype are not PHSCs.33,34 Some cells with this phenotype only provide short-term hematopoietic repopulation and some have no demonstrable stem cell activity.35 Some of the cells that we have grown can function as stem cells. Cells capable of both short-term (3-4 week) and long-term (30 week) repopulation are present and appear to have undergone a significant increase in number. The Sca-1+/LIN- cells that we sorted from catalase-treated LTBMCs are both phenotypically and functionally diverse. Only a small proportion (490/105) produce colonies in methyl cellulose (Figure 7B). Among the remainder are stem cells capable of long-term multilineage reconstitution and secondary transfer as well as short-term bone marrow repopulation. Within the total Sca-1+/LIN- population we also identified a population that coexpresses c-Kit. In mice, this is the phenotype used to identify PHSCs.23,24 Many of these Sca-1+/LIN- cells appear to be in the endothelial lineage. The photomicrographs shown in Figure 6 indicate that in the presence of catalase, the low-density BM cells used to initiate hematopoiesis in these cultures can give rise to adherent cells of the stromal layer. These cells are CD45- and CD31+. Tie-2 is also expressed by CD45+ cells and these cells also express CD31 and Sca-1+. Tie-2 expression is highly correlated with CD34 expression. In the mouse, CD34 is primarily an endothelial cell marker and is expressed on only a small subset of hematopoietic cells.36,37 A similar correlation was found with KDR (VEGF2r) expression (data not shown). These results suggest the possibility that these nonadherent cells may be the equivalent of circulating endothelial lineage cells in various stages of maturation and that at least some of them may be hemangioblasts. It is also possible that the development of an adherent layer of CD31+ cells may contribute to the formation of a microenvironment that supports hematopoiesis.
Multilineage reconstitution of myeloablated mice by BM cells cultured with catalase 6 weeks after transplantation. Fluorescence-activated cell sorter (FACS) analysis demonstrating multilineage engraftment of donor-derived (CD45.1+) cells in the peripheral blood that are stained with Gr-1-PE and Mac-1-FITC (left) and B220-PE and CD3-FITC (right).
Multilineage reconstitution of myeloablated mice by BM cells cultured with catalase 6 weeks after transplantation. Fluorescence-activated cell sorter (FACS) analysis demonstrating multilineage engraftment of donor-derived (CD45.1+) cells in the peripheral blood that are stained with Gr-1-PE and Mac-1-FITC (left) and B220-PE and CD3-FITC (right).
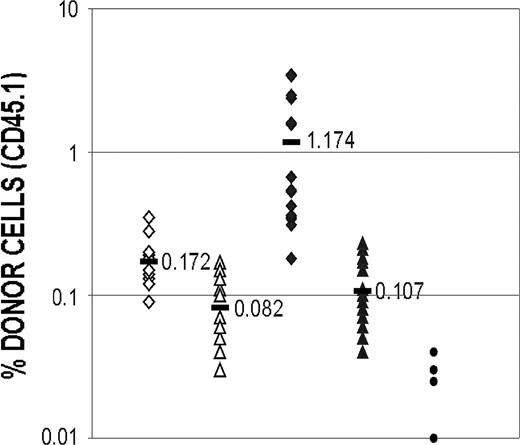
Reconstitution of myeloablated mice after secondary transfer of BM cells obtained from mice that had previously been grafted with cultured cells. Mice that had received a transplant with either catalase-cultured cells or control cells were killed 30 weeks after transplantation and BM cells prepared. Cells from each group of mice were pooled and one million viable BM cells were transplanted into irradiated C57Bl/6 (Ly5.2) recipients as previously described. After 1 month the recipients were bled and the proportion of culture-derived (Ly5.1) cells in the peripheral blood of each mouse was determined. The difference between the proportion of cultured cells recovered from the catalase-treated and control cultures after the injection of 50 000 cells is significant with a P value of less than .001 (Student t test; 2-tailed, unpaired samples). ⋄ indicates 50 000 cells from control culture; ▵, 20 000 cells from control culture; ♦, 50 000 cells from catalase culture; ▴, 20 000 cells from catalase culture; •, background; and —, mean.
Reconstitution of myeloablated mice after secondary transfer of BM cells obtained from mice that had previously been grafted with cultured cells. Mice that had received a transplant with either catalase-cultured cells or control cells were killed 30 weeks after transplantation and BM cells prepared. Cells from each group of mice were pooled and one million viable BM cells were transplanted into irradiated C57Bl/6 (Ly5.2) recipients as previously described. After 1 month the recipients were bled and the proportion of culture-derived (Ly5.1) cells in the peripheral blood of each mouse was determined. The difference between the proportion of cultured cells recovered from the catalase-treated and control cultures after the injection of 50 000 cells is significant with a P value of less than .001 (Student t test; 2-tailed, unpaired samples). ⋄ indicates 50 000 cells from control culture; ▵, 20 000 cells from control culture; ♦, 50 000 cells from catalase culture; ▴, 20 000 cells from catalase culture; •, background; and —, mean.
After 4 to 5 weeks the catalase-treated cultures stop releasing differentiated hematopoietic cells into the culture medium and both progenitors and cells with a stem cell phenotype decline. After 4 to 5 weeks catalase-treated cultures resemble aging conventional long-term BM cultures. Surprisingly, if the catalase is removed at this time, normal hematopoiesis is restored. The recovery is rapid and within 7 to 10 days the cultures are indistinguishable from those that had never been treated with catalase. This rapid recovery indicates that the absence of stem cell activity is not the result of toxicity; catalase treatment does not eliminate HSCs but, rather, renders them inactive or quiescent. Long-term culture-initiating cells (LTC-ICs) have been shown to enter this state of quiescence spontaneously38 and our results suggest that peroxides play a role in the development of this state.
It is likely that the effect of catalase is primarily on the added low-density bone marrow cells in the culture and not upon the preformed stromal layer. Stromal cultures that had been irradiated or mitomycin C treated and thus rendered incapable of new message production were still able to show the effects of catalase. Furthermore, since hematopoietic progenitors are increased in both the adherent and nonadherent compartments, redistribution of progenitors is not the explanation for the catalase effect.
Neither the processes that determine whether a stem cell undergoes lineage commitment or self-renewal or the ones that govern the progress from progenitor to mature effector cell are fully understood. The results show that catalase alters this pathway and suggest that several steps in the pathway are subject to ROS regulation. Thus, neutrophil production in the culture begins immediately (Figure 1A) but declines to below control levels at a time that progenitors (CFU-Cs) are increasing (Figure 1D). In a similar fashion CFU-Cs decline to below control levels (Figure 1D) at a time when long-term repopulating cells are more abundant in the catalase-treated cultures than in the controls (Table 4). How catalase mediates these apparently contradictory effects is not clear. It is possible that catalase either induces the production of new cytokines or prevents the degradation of those already present but specific targets are difficult to identify. Medium from catalase-treated cultures does not contain increased levels of colony-stimulating factors (data not shown), and none of the known growth factors, when added alone, are mitogenic for HSCs.39-43 It has been suggested that the role of the known lineage-specific cytokines is to abet the survival of stem cells44 rather than to induce their differentiation. It has also been demonstrated that suppression of apoptosis allows differentiation and development of a multipotent hematopoietic cell line in the absence of added growth factors.45 The effect of catalase may be to promote stem cell and progenitor survival by inhibiting both differentiation and apoptosis.
It is possible that the hematopoietic effects are caused by alterations in the supportive stroma. Our data show that catalase treatment leads to the development of endothelial-like cells in the adherent layer. These cells may be able to provide the equivalent of the “stromal niche” that is believed to be required for HSC survival.46
A direct effect of catalase on the intracellular redox potential might also be responsible for the observed effects. Exogenously supplied catalase may enter cells and alter activity of redox-sensitive protein phosphatases and transcription factors. Proteins in the medium enter cells by a variety of pathways but none of these are efficient in transferring intact proteins into the cytosol in the absence of receptor binding.47-49 This barrier may explain the relatively high concentration of catalase that is required to alter hematopoiesis in culture. Watanabe et al50 demonstrated that catalase, complexed with the HIV viral product TAT, could enter cells and inhibit serum-induced Elk phosphorylation and anisomycin- and/or MG-132-induced ERK phosphorylation. They showed that free catalase entered cells less well than the complex but both appeared to bind to cell membranes.
Alternatively, catalase may be acting on peroxides released into the medium by the cells growing in the cultures. Peroxides are freely diffusible and at the appropriate concentration they induce apoptosis rather than necrosis. Proapoptotic reactive oxygen intermediates derived from mature phagocytic cells have been shown to play a role in limiting progenitor cell self-renewal.10 Many growth factors appear to promote cell growth by providing an antiapoptotic signal51-55 and it seems possible that catalase, by removing an apoptotic signal, mimics these growth factors. Agents that reduce ROS levels have been shown to be antiapoptotic.56,57
The fact that catalase profoundly alters hematopoiesis adds to the growing body of evidence that redox signals play an important role in many differentiating systems. H2O2 and other ROSs are known to function in signal transduction pathways inducing cell death, cell survival, gene activation, and cell movement,58-60 and activation with GM-CSF and other growth factors including IL-3, SF, and TPO has been shown to be associated with alterations in levels of H2O2.61,62
It is intriguing how well catalase treatment replicates the effects of the so-called stem cell niche of the bone marrow, promoting myeloid differentiation without lymphopoiesis and inducing a quiescent state in PHSCs.63,64 In addition to highlighting the importance of peroxide levels in regulating hematopoietic differentiation, these cultures may provide a unique source of both stem cells and committed but undifferentiated hematopoietic progenitors. These Sca-1+/LIN- progenitors have both serologic and functional properties that indicate that this population is highly enriched for both HSCs and cells of the endothelial lineage. While considerable success has been achieved in growing stem cells from mice that have been genetically modified,65-68 unmodified cells have proved more difficult to grow. The best results reported previously are the 4- to 6-fold expansion achieved using human TAT-HOXB4 protein69 and a similar expansion of severe combined immunodeficiency (SCID)-repopulating cells in cord blood cells cultured with a copper chelator.70 Our results indicate that a greater expansion is possible if the redox environment of the cells is modified.
Prepublished online as Blood First Edition Paper, November 8, 2005; DOI 10.1182/blood-2005-03-1180.
Supported by National Institutes of Health (NIH) grants R01 HL073713 (R.S.B.) and DA043152 (S.K.). R.G. is supported by NIH Hematology Training Grant 2T32 Hl007151-26. The Flow Cytometry Laboratory is supported by the NYU Cancer Center Core Grant (P30 CA 16097-21).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The skillful assistance of Dr John Hirst of the NYU-CI Flow Cytometry Core Facility is gratefully acknowledged.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal