Patients with t(8;21) acute myeloid leukemia (AML) are considered to have a good prognosis; however, approximately 50% of them relapse. The genetic alterations associated with a poor outcome in t(8;21) AML remain unknown. Recently, aberrations of receptor tyrosine kinases (RTKs) were frequently found in patients with AML. However, the prevalence and prognostic impact of RTK aberrations in pediatric t(8;21) AML remains undetermined. Here, we found the kinase domain mutations of the KIT gene in 8 (17.4%) of 46 patients with t(8;21) AML among newly diagnosed pediatric patients with AML treated on the AML99 protocol in Japan. Significant differences between patients with or without KIT mutations were observed in the 4-year overall survival (50.0% versus 97.4%, P = .001), disease-free survival (37.5% versus 94.7%, P < .001) and relapse rate (47.0% versus 2.7%, P < .001). Furthermore, FLT3 internal tandem duplication was found in only 2 (4.3%) patients. These results suggested that KIT mutations are strongly associated with a poor prognosis in pediatric t(8;21) AML.
Introduction
Patients with t(8;21) acute myeloid leukemia (AML) have been reported to have a good prognosis; however, approximately 50% of them relapse.1,2 A high presenting leukocyte count, CD56 expression, or extramedullary disease has been reported to be associated with a poor prognosis in t(8;21) AML.1,3,4 However, the genetic alterations associated with a poor outcome in patients with t(8;21) AML remain unknown. Recent studies revealed that internal tandem duplication (ITD) of FLT3 is considered to be one factor predicting poor prognosis in adult and pediatric patients with AML.5-9 More recently, KIT mutations were found in 12.7% to 48.1% of adult patients with AML with t(8;21)10-12 and were reported to be associated with a poor prognosis.13,14 The prevalence and prognostic impact of KIT mutations in pediatric t(8;21) AML remain unknown. We performed the mutational analysis of KIT and FLT3 in pediatric patients with t(8;21) AML who were treated on the Japanese Childhood AML Cooperative Study Group Protocol, AML99. We report here that KIT mutations are strongly associated with a poor prognosis in pediatric patients with t(8;21) AML.
Study design
Patients and samples
The diagnosis of AML was based on the French-American-British (FAB) classification, and cytogenetic analysis was performed using a routine G-banding method. From January 2000 to December 2002, 318 patients were newly diagnosed as having de novo AML. Of 240 patients, 77 (32.1%), except for 29 AML-M3 and 49 Down syndrome, had t(8;21)(q22; q22) according to cytogenetics or AML1-MTG8 fusion transcript with the reverse-transcriptase-polymerase chain reaction (RT-PCR) (Figure S1; see the Supplemental Materials link at the top of the online article, at the Blood website). Samples were available from 135 (56.3%) of 240 patients with AML, including 46 (59.7%) of 77 patients with t(8;21) AML. Of 46 patients with t(8;21) AML, 3 patients were classified into M1, 39 into M2, and 4 into M4. There were no statistical differences between 46 analyzed patients with t(8;21) AML and the 31 nonanalyzed patients in age (median 7.5 years [range: 2-15 years] versus 9 years [range: 1-15 years]), initial white blood cell (WBC) count (median: 14.4 × 109/L; range: 1.65 × 109/L-107.7 × 109/L; versus 9.1 × 109/L; range: 1.4 × 109/L-136 × 109/L), induction rate (100% versus 93.5%), relapse rate (15.2% versus 19.4%), and 4-year overall survival rate (4y-OS; 87% versus 91%). In the AML99 protocol, patients with t(8;21) with initial WBC count lower than 50 × 109/L were categorized into a low-risk group. Thus, after patients with t(8;21) AML obtained complete remission (CR) with induction chemotherapy (cytarabine, etoposide, and mitoxantrone), they were treated with 5 additional courses of intensive chemotherapy (high-dose cytarabine [HDCA], etoposide, idarubicine, and mitoxantron; Figure S2 and Tsukimoto et al15 ). If the initial WBC count was greater than 50 × 109/L, patients were categorized into an intermediate-risk group and received allogeneic stem cell transplantation (allo-SCT) in the case of the presence of a donor. Informed consent was obtained from the patients or patients' parents, according to guidelines based on the tenets of the revised Helsinki protocol. The institutional review board of Gunma Children's Medical Center approved this project.
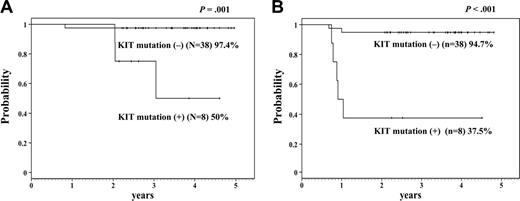
Kaplan-Meier analysis. This analysis shows 4-year overall survival (A) and disease-free survival (B) of the patients with or without KIT mutation. The difference is statistically significant (A: P = .001; B: P < .001).
Kaplan-Meier analysis. This analysis shows 4-year overall survival (A) and disease-free survival (B) of the patients with or without KIT mutation. The difference is statistically significant (A: P = .001; B: P < .001).
KIT mutation analysis
Mutational analysis of the extracellular (EC) domain (exons 8, 9), transmembrane (TM) domain (exon 10), juxtamembrane (JM) domain (exon 11), and the second intracellular kinase (TK) 2 domain (exons 17 and 18) of KIT gene was performed with RT-PCR followed by direct sequencing. Primers used are shown in Table S1.
FLT3 mutation analysis
Statistical analysis
Estimation of survival distributions was performed using the Kaplan-Meier method and the differences were compared using the log-rank test. Disease-free survival (DFS), event-free survival (EFS), and overall survival (OS) were defined as the times from diagnosis to relapse, from diagnosis to event (relapse or death of any cause), and from diagnosis to death of any cause or the last follow-up. Statistical difference analysis was performed using the χ2 test.
Results and discussion
KIT and FLT3 expressions were found in all of the 46 t(8;21) AML samples. Although KIT mutations have been reported in a small number of pediatric patients with t(8;21) AML,8,19 TK2 domain mutations of the KIT gene were found in 8 (17.4%) of 46 patients in this study (Table 1). However, we could not find any mutation other than the TK2 domain. The N822K mutation, which has been frequently reported so far,12 was found in 3 of 8 patients in this study.
Clinical characteristics of patients with t(8;21) AML with KIT mutations
Patient no. . | Age, y . | Sex . | WBC count, × 109 cells/L . | Additional chromosome abnormalities . | Time of relapse, mo . | Status of allo-SCT . | Survival, mo . | KIT mutation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 14.10 | None | 12 | Second CR | 37 | A814S |
| 2 | 8 | M | 27.60 | –Y | 14 | Second CR | 47* | N822K |
| 3 | 8 | F | 10.77 | –X | 10 | Second CR | 25 | D816H |
| 4 | 6 | M | 34.50 | –Y, +4 | 12 | Second CR | 26* | N822K |
| 5 | 3 | F | 20.50 | None | 11 | — | 25 | N822K |
| 6 | 1 | F | 4.60 | –X, t(7;9) | — | — | 32* | N822T |
| 7 | 15 | M | 20.80 | –Y | — | First CR | 56* | D816V |
| 8 | 13 | M | 66.20 | None | — | First CR | 30* | V825A |
Patient no. . | Age, y . | Sex . | WBC count, × 109 cells/L . | Additional chromosome abnormalities . | Time of relapse, mo . | Status of allo-SCT . | Survival, mo . | KIT mutation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | F | 14.10 | None | 12 | Second CR | 37 | A814S |
| 2 | 8 | M | 27.60 | –Y | 14 | Second CR | 47* | N822K |
| 3 | 8 | F | 10.77 | –X | 10 | Second CR | 25 | D816H |
| 4 | 6 | M | 34.50 | –Y, +4 | 12 | Second CR | 26* | N822K |
| 5 | 3 | F | 20.50 | None | 11 | — | 25 | N822K |
| 6 | 1 | F | 4.60 | –X, t(7;9) | — | — | 32* | N822T |
| 7 | 15 | M | 20.80 | –Y | — | First CR | 56* | D816V |
| 8 | 13 | M | 66.20 | None | — | First CR | 30* | V825A |
— indicates not applicable.
Patient still alive
The statistical differences between patients with or without KIT mutations were not significant in age (median 8 years [range: 1-15 years] versus 7 years [range: 2-15 years]), and the initial WBC count (median: 20.65 × 109/L; range: 4.6 × 109/L-66.2 × 109/L; versus 14.3 × 109/L; range: 1.65 × 109/L-107.7 × 109/L). Interestingly, KIT mutations were observed only in M2 patients according to FAB classification. Another report also suggested that KIT mutations were frequently found in M2 patients with t(8;21).19 Significant differences between patients with or without KIT mutations were observed in 4-year OS (50.0% versus 97.4%, P = .001, Figure 1), DFS (37.5% versus 94.7%, P < .001), and relapse rate (47.0% versus 2.7%, P < .001). Short CR duration and high relapse rate were more significant than those of the previous report in adults.14 KIT mutations have recently been reported not to influence the clinical outcome in pediatric core-binding factor (CBF) leukemia patients.20 Although they found KIT mutations in 5 of 16 cases of t(8;21) AML, they did not describe the clinical outcome of patients with t(8;21) AML with or without KIT mutations. Furthermore, the clinical outcome of the patients without KIT mutations in their study was poorer than the outcome of those in our study (EFS 63% versus 92.1%). Our result may depend on our good clinical outcome of patients with t(8;21) AML without KIT mutations.
Except for 2 patients who received allo-SCT in first CR (patients no. 7 and no. 8 in Table 1), 5 of 6 (83.3%) patients with the mutation relapsed within 14 months after diagnosis. Allo-SCT was performed in 6 of 8 patients with t(8;21) AML with KIT mutations (2 in first CR, 4 in second CR) and 4 patients are still alive. In contrast, allo-SCT was also performed in only 1 of 38 patients with t(8;21) AML without KIT mutation in second CR, and this patient is still alive.
A high presenting leukocyte count and extramedullary disease were not associated with the poor prognosis in this study. Notably, KIT was mapped to chromosome 4 at band q11 and trisomy 4 was reported to be associated with KIT mutation.21 One patient with trisomy 4 in addition to t(8;21) had N822K mutation (patient no. 4). As for additional chromosome abnormality, loss of sex chromosome was observed in 5 (62.5%) of 8 patients with KIT mutation and 14 (37%) of 38 patients without mutations, although the difference between them was not statistically significant. Recently, it has been reported that AML blasts with N822K mutation are sensitive to the tyrosine kinase inhibitor Gleevec/STI571/imatinib mesylate.12 The effectiveness of imatinib mesylate for the patient with AML with KIT mutation was also reported.22 Thus, tyrosine kinase inhibitors may be applicable for these patients in the future.
Two samples examined at relapse showed the same mutations as those at diagnosis (patients no. 3 and no. 5), and these KIT mutations disappeared in samples in remission, suggesting that KIT mutation was not a constitutional abnormality.
Recently, clonal leukemic cells with AML1-MTG8 fusion transcript have been reported to arise in utero.23 Moreover, it was reported that this fusion transcript was not sufficient for full leukemogenesis, and that additional genetic events were required.24,25 KIT mutations may be one of the secondary genetic events of the stepwise leukemogenesis of t(8;21) AML.
FLT3-ITD was found in only 2 (4.6%) of 46 patients with t(8;21). One patient died during chemotherapy, and the other patient was disease free for 42 months from diagnosis. FLT3-ITD is considered to be strongly associated with a poor prognosis in AML.6,7 However, FLT3-ITD was rarely reported in patients with t(8;21) AML.8,9,13,14,20 Our data also confirmed the low incidence of FLT3-ITD in patients with t(8;21) AML. As for D835Mt of the FLT3 gene, we found the mutation in 1 of 46 patients, who was alive for 31 months after diagnosis.
In total, 11 (23.9%) of 46 patients with t(8;21) AML in this study had KIT or FLT3 mutations, suggesting that the pediatric patients with t(8;21) AML had genetic heterogeneity. In conclusion, KIT mutations are considered to be strongly associated with poor prognosis in pediatric t(8;21) AML.
Appendix
Members of the Japanese Childhood AML Cooperative Study Group who contributed data to the study include Akira Morimoto, Department of Pediatrics, Kyoto Prefectural University of Medicine; Ryoji Kobayashi, Department of Pediatrics, Hokkaido University School of Medicine; Hiromasa Yabe, Department of Pediatrics, Tokai University School of Medicine; Kazuko Hamamoto, Department of Pediatrics, Hiroshima Red Cross Hospital; Shigeru Tsuchiya, Department of Pediatric Oncology, Institute of Development, Aging, and Cancer, Tohoku University; Yuichi Akiyama, Department of Pediatrics, National Hospital Organization Kyoto Medical Center; Hisato Kigasawa, Department of Hematology, Kanagawa Children's Medical Center; Akira Ohara, Department of First Pediatrics, Toho University School of Medicine; Hideki Nakayama, Department of Pediatrics, Hamanomachi Hospital; Kazuko Kudo, Department of Pediatrics, Nagoya University Graduate School of Medicine; and Masue Imaizumi, Department of Hematology/Oncology, Miyagi Prefectural Children's Hospital.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-08-3408.
A list of the participating members of the Japanese Childhood AML Cooperative Study Group appears in “Appendix.”
Supported in part by a Grant-in-Aid for Cancer Research and a grant for Clinical Cancer Research from the Ministry of Health, Labor, and Welfare of Japan, and by a research grant for Gunma Prefectural Hospitals.
A.S. performed genetic analysis and wrote the paper; T.T. assisted with the genetic analysis; K.T. performed the statistical analysis; A.T., K.H., M.T., and R.H. arranged the clinical data; I.T. designed the AML cooperative study in Japan; and Y.H. designed the study and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to all members of the Japanese Childhood AML Cooperative Study Group.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal