Comment on Zhao et al, page 2138
A novel isoform of the erythroid-specific NF-E4 protein contributes to fetal globin gene silencing by sequestering the CP2 transcriptional activator away from the γ-globin gene promoter.
During the embryonic, fetal, and adult stages of hematopoiesis, distinct patterns of globin protein production result from the sequential activation and silencing of genes within the α- and β-globin gene loci. Of these changes, the switch from fetal γ-globin expression to adult β-globin expression has been the most studied. The primary rationale for focusing on this area is that if the γ-globin genes could be reactivated in patients with sickle cell disease or β-thalassemia, then the hemoglobin sickling or globin chain imbalance associated with these diseases could be lessened and disease severity mitigated.1,2
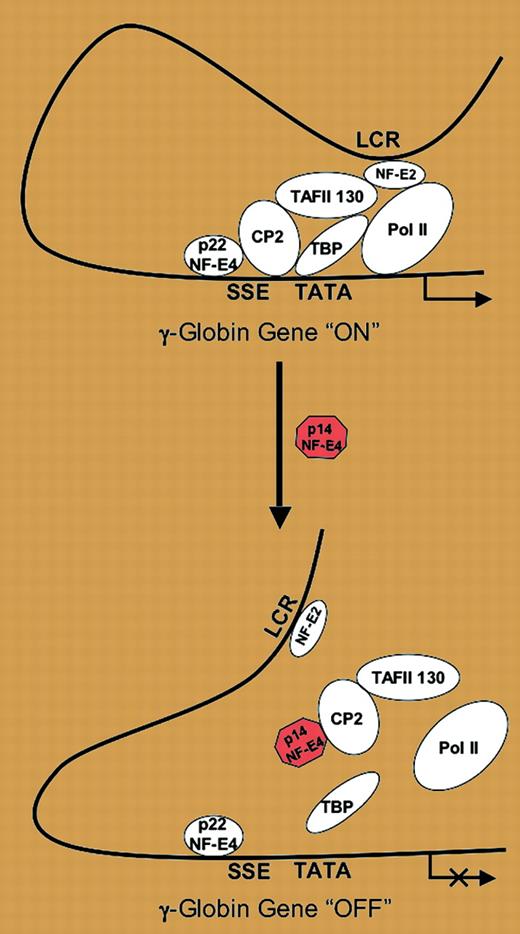
Model of p14 NF-E4 repression of γ-globin gene expression. See the complete figure in the article beginning on page 2138.
Model of p14 NF-E4 repression of γ-globin gene expression. See the complete figure in the article beginning on page 2138.
The report by Zhao and colleagues in this issue of Blood provides evidence supporting a new mechanism for one component of the γ-globin to β-globin switch, the silencing of the fetal gene. The report is based on a prior description by the same group of an element in the proximal γ-globin promoter termed the “stage selector element,” or SSE. They have previously shown that a protein complex, the “stage selector protein,” or SSP, that binds to this element is composed of a ubiquitous transcriptional activator, CP2, and a 22-kDa erythroid protein, NF-E4, and that this complex acts at the SSE to stimulate γ-globin gene expression in fetal erythroid cells.3 Earlier in their investigations, the group proposed that methylation of CpG dinucleotides within the SSE in adult erythroid cells prevented the activating complex from binding, resulting in silencing of the fetal gene. The authors now implicate a 14-kDa “short form” of the NF-E4 protein, which appears to be increased in adult erythroid cells, in a similar process. First, they show that the 14-kDa form of NF-E4 results from the use of an alternative translational start site. Next, they demonstrate that while the smaller NF-E4 protein is still able to form a complex with CP2, overexpression of the 14-kDa NF-E4 isoform in K562 cells and erythroid cells differentiated in vitro from cord blood CD34+ cells results in significantly decreased γ-globin gene expression. This decreased expression is associated with the loss of CP2, NF-E4, and RNA polymerase from the γ-globin promoter. Expression of a mutant 14-kDa NF-E4 that does not interact with CP2 fails to suppress γ-globin production. On the basis of this information, the authors propose that γ-globin silencing is the result of the short-form NF-E4 sequestering CP2 away from the promoter. This model is depicted in the figure.
While this article makes a strong case for a new γ-globin silencing mechanism, as always, additional questions remain. For example, this mechanism will need to be evaluated in primary human erythroid cells, where developmental changes in γ-globin expression physiologically occur. In addition, a strategy to specifically inhibit production or function of short-form NF-E4 in a developmental model would allow the role the protein plays in switching to be more precisely determined. Finally, it would be of interest to know whether such inhibition in adult erythroid cells would reactivate γ-globin chain production, thus identifying this protein as a potential therapeutic target. Despite nearly 30 years of molecular biology research, a comprehensive model of globin gene regulation remains elusive.4 Zhao and colleagues have provided another piece of this complex puzzle. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal