Comment on Hayhoe et al, page 2123
The glucocorticoid-induced annexin 1 system controls neutrophil trafficking. Hayhoe and colleagues report the receptors and anti-inflammatory mechanisms for annexin 1 and its N-terminal peptide Ac2-26 that limit neutrophil rolling, adhesion, and diapedesis.
Although the unification hypothesis for aspirin and aspirin-like drugs as inhibitors of cyclooxygenases introduced by Vane in 19711 offered answers to the long-sought mechanism of action for aspirin (synthesized in 1897), the means by which glucocorticoids regulate inflammation and leukocyte infiltration still remained to be reconciled (reviewed in Flower2).1 Glucocorticoids are among the most potent and widely used anti-inflammatory drugs and have no direct actions on cyclooxygenases as indicated in the earlier work of Sir John Vane, Rod Flower, and others.2 Glucocorticoids stimulate the appearance of lipocortin (also known as annexin 1, abbreviated ANXA1 in today's literature), which down-regulates leukocytes, constituting some of the anti-inflammatory properties of glucocorticoids.3 Yet the direct molecular mechanisms by which ANXA1 regulates inflammation remained of interest. In this issue, Hayhoe and colleagues demonstrate, for the first time, that the full-length ANXA1 and its N-terminal peptide (Ac2-26) each initiate different mechanisms or cellular “fingerprints” that regulate neutrophil extravasation via directly interacting with specific cell surface receptors of the G-protein-coupled receptor (GPCR) type.
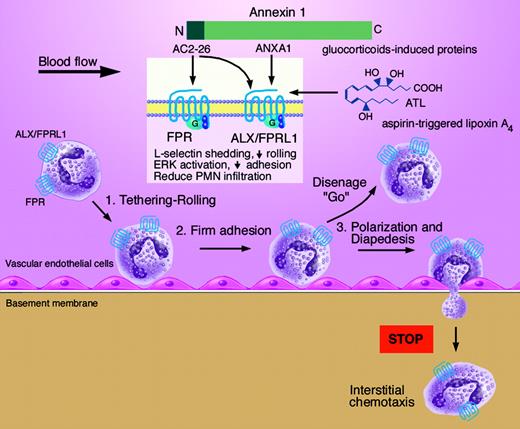
Neutrophil migration through vessels involves several stages of interactions with endothelium: tethering-rolling, stable adhesion, and diapedesis. Annexin 1 (ANXA1) and its N-terminal peptide (Ac2-26) directly interact with 2 G-protein-coupled receptors (FPR and ALX/FPRL1) to halt neutrophil diapedesis. Glucocorticoid-stimulated proteins (ANXA1 and Ac2-26), as well as aspirin-triggered lipoxin A4 (ATL), converge at the same receptor ALX/FPRL1 to both control excessive neutrophil infiltration and limit inflammatory responses. Illustration by Marie Dauenheimer.
Neutrophil migration through vessels involves several stages of interactions with endothelium: tethering-rolling, stable adhesion, and diapedesis. Annexin 1 (ANXA1) and its N-terminal peptide (Ac2-26) directly interact with 2 G-protein-coupled receptors (FPR and ALX/FPRL1) to halt neutrophil diapedesis. Glucocorticoid-stimulated proteins (ANXA1 and Ac2-26), as well as aspirin-triggered lipoxin A4 (ATL), converge at the same receptor ALX/FPRL1 to both control excessive neutrophil infiltration and limit inflammatory responses. Illustration by Marie Dauenheimer.
Leukocyte extravasation requires at least several critical stages of endothelium interactions: tethering-rolling, firm adhesion, and diapedesis (see figure). Ac2-26 attenuates neutrophil rolling by initiating l-selectin shedding, and ANXA1 reduces adhesion. These specific actions are explained by interactions with 2 different members of the formyl peptide receptor family: formyl peptide receptor (FPR) and the lipoxin A4 receptor/formyl peptide receptors-like 1 (ALX/FPRL1). In this issue, Hayhoe and colleagues establish that ANXA1 specifically binds to ALX-FPRL1, and the peptide Ac2-26 binds to both FPR and ALX-FPRL1, which was demonstrated using specific radioligand binding and intracellular ERK activation. Thus, the differential temporal and spatial actions elicited by ANXA1 and its Ac2-26 peptide in tandem identify candidate mechanisms by which glucocorticoids regulate neutrophils.
The first member of the formyl peptide receptor family, namely FPR, was identified and cloned in 1990 as a G-protein-coupled receptor. It binds bacterial-derived formyl peptides (such as the surrogate peptide fMLP) with high-affinity activating phagocytes. Two other members were identified using low-stringency cross-hybridization with human FPR and, based only on nucleotide sequence homology, named FPR-like-1 and FPR-like-2. However, FPRL1 binds fMLP with only very low affinity, and no other functional responses for FPRL1 were identified. Later, an endogenous lipid mediator, lipoxin A4 (LXA4), and its aspirin-triggered epimer 15-epi-LXA4 (ATL), displayed high affinity to FPRL1, triggering anti-inflammatory signals. Thus, this previous orphan was termed ALX/FPRL1. In addition to its lipid-derived ligands, many peptides/proteins interact at least in vitro with ALX/FPRL1 that evoke distinct downstream signaling.4,5 These include bacteria- and host-derived peptides, HIV envelope proteins/peptides, and neurotoxic as well as synthetic peptides (recently reviewed in Le et al6 ). Among them, ANXA1 and Ac2-26 are to date the only “anti-inflammatory” proteins/peptides that interact directly with human ALX, joining lipoxin's ability to halt neutrophil diapedesis via this receptor.
The 2 most widely used and successful anti-inflammatory drugs, glucocorticoids and aspirin, each trigger distinct classes of endogenous agonists (ANXA1 and ATL) that converge at this same receptor, namely ALX/FPRL1. In this elegant act of nature, both proteins and small lipid-derived mediators use this theme as endogenous counterregulatory agonists to control neutrophils during inflammation. ANXA1 mimetics that attenuate neutrophil functions via ALX may reach from bench to clinic to limit overwhelming inflammation in human diseases, as is the case with LXA4, ATL, and their analogs. These glucocorticoid- and aspirin-triggered pathways likely represent redundancies in endogenous anti-inflammation circuits that open avenues for new therapeutic approaches to manage the unwanted side effects of inflammation and neutrophil-mediated tissue injury. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal