A common point mutation in a conserved negative regulatory domain of the JAK2 (Janus kinase 2) tyrosine kinase leads to constitutive hematopoietic growth factor receptor signaling and was recently described in many patients with myeloproliferative disorders (MPDs), especially polycythemia vera.1-4 However, this JAK2 mutation is present in only a subset (35%-50%) of patients with myelofibrosis with myeloid metaplasia (MMM) or essential thrombocythemia, and it is rare in atypical MPDs, myelodysplastic syndrome, or other neoplastic myeloid disorders.5-7 Even when present, the JAK2 mutation is associated with phenotypic diversity. Cooperating mutations or distinct genetic/cellular background must be responsible for the variety of myeloid neoplasms associated with JAK2, yet such mutations have not yet been described, and the proliferative signals responsible for MPDs in the absence of JAK2 mutations remain largely unknown.
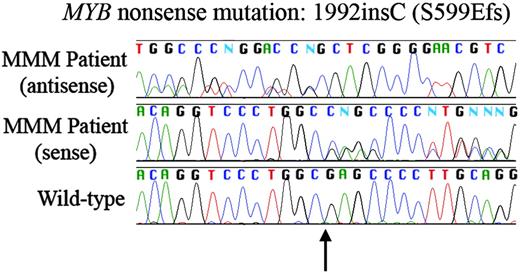
Frameshifting point mutation in MYB gene in granulocytic DNA from a patient with myelofibrosis with myeloid metaplasia. Insertion of a cytosine base (arrow; c.1992insC and p.S599EfsX605; GenBank reference sequences NM_005375 and NP_005366, respectively) leads to a frameshift with premature stop codon generation. This was the only nonsynonymous point mutation detected in analysis of MYB gene (25 patients) and EP300 KIX domain. Bottom: wild-type; middle: patient sense sequence; top: patient antisense sequence. Patient sequences demonstrate confused sequence expected for mixed clonality insertional mutation.
Frameshifting point mutation in MYB gene in granulocytic DNA from a patient with myelofibrosis with myeloid metaplasia. Insertion of a cytosine base (arrow; c.1992insC and p.S599EfsX605; GenBank reference sequences NM_005375 and NP_005366, respectively) leads to a frameshift with premature stop codon generation. This was the only nonsynonymous point mutation detected in analysis of MYB gene (25 patients) and EP300 KIX domain. Bottom: wild-type; middle: patient sense sequence; top: patient antisense sequence. Patient sequences demonstrate confused sequence expected for mixed clonality insertional mutation.
We read with great interest the recent reports8,9 of mice with homozygous point mutations in either the DNA binding domain or leucine zipper domain of the c-Myb (v-avian myeloblastosis virus oncogene homolog) transcription factor. These mice develop thrombocytosis on either thrombopoietin (TPO) receptor–deficient or wild-type backgrounds, as do patients with MMM. An eerily similar phenotype arises in mice with homozygous germ-line point mutations in the c-Myb binding surface (KIX domain) of the p300 transcription coactivator.10 The key role of c-Myb and p300 in normal hematopoiesis is also demonstrated by embryonic lethality of murine knock-out models due to failure of definitive hematopoiesis,11 as well as the apparent requirement for c-Myb down-regulation for normal thrombocyte development.12 In both p300 and c-Myb mutant mice, disordered megakaryocytopoiesis leads to marrow and spleen hypercellularity, peripheral blood thrombocytosis independent of thrombopoietin signaling, erythroid hypoplasia and anemia, and various abnormalities of lymphocyte development—all prominent characteristics in some forms of human MPDs, especially MMM. Therefore, MYB and EP300 (E1A binding protein p300)—the human homologs of genes encoding murine c-Myb and p300, respectively—are attractive candidate genes for human MPD.
After study approval by the Mayo Clinic institutional review board, we analyzed peripheral blood granulocyte genomic DNA from 25 patients with cellular or fibrotic MMM for mutations in the coding region of MYB (all 15 exons) and analyzed 30 additional patients (10 MMM, 10 with essential thrombocythemia, and 10 with polycythemia vera) for mutations in the region of EP300 that encodes the KIX domain. All 55 patients fit established World Health Organization diagnostic criteria and gave written informed consent for molecular analysis of blood samples. We used polymerase chain reaction (PCR) and standard automated fluorescent dye chemistry DNA sequencing (primers and amplification protocols available from the authors on request), supplemented by denaturing high-performance liquid chromatography to detect mutations with mixed clonality. The analysis was complemented by expression analysis by real-time PCR where mRNA was available.
EP300 was unremarkable in all 30 patients tested. A single MYB heterozygous/mixed clonality nonsense mutation (c.1992insC and protein [p].S599EfsX605; GenBank reference sequences NM_005375 and NP_005366, respectively)16 was found in a fibrotic MMM sample (Figure 1). Buccal cells were not available from this patient to confirm whether the mutation was somatic or germ line; regardless, it may not be pathogenic, as the leucine zipper, interaction, and regulatory domains are all N-terminal— there are normal Myb isoforms in which all residues C-terminal to 351 (SwissProt isoform 3) are truncated and others in which residues carboxy-terminal to 567 are altered (isoform 6). The change was not present in 25 healthy controls (Continental European, Icelandic, and African-Jamaican DNA). We also detected a few synonymous single nucleotide polymorphisms in MYB, all also present in samples from healthy persons. Expression of MYB at the mRNA level did not differ significantly between MPD patients and healthy or anemic (non-MPD) controls. We conclude that point mutations in the coding region of MYB and P300 may cause murine myeloproliferation, but are probably not common in human classical MPDs. While the c-Myb and p300 mutant mice appear to be excellent systems for studying mammalian hematopoiesis, they may have limitations as human disease models, similar to GATA1low mice or TPO-overexpressing mice.13-15
Point mutations in MYB and the KIX domain of EP300 are uncommon in the human myeloproliferative disorders
In mice,1-4 mutations in the genes encoding c-Myb or its partner protein p300 alter lineage specification of hematopoietic cells resulting in increased megakaryocytopoiesis that is independent of thrombopoietin signaling.
Given the phenotypic similarities between these mutant mice and patients with essential thrombocythemia (ET) and idiopathic myelofibrosis (IM), we hypothesized that alterations in these genes may contribute to human myeloproliferative disorders (MPDs), particularly those with excessive thrombopoiesis, either in isolation or in concert with the recently described JAK2V617F mutation.5-8 To address this, we performed sequence analysis on DNA from purified peripheral blood granulocytes in 52 patients with myeloproliferative diseases. All subjects provided written informed consent and the study was approved by the Melbourne Health Human Research Ethics Committee. Thirty-eight of these patients were diagnosed with ET (55% were JAK2V617F positive based on allele-specific polymerase chain reaction [PCR]5 ), 9 with polycythemia vera (PRV; 100% JAK2V617F positive), and 5 patients with IM (60% JAK2V617F positive). The coding region and canonical splice sites of the 15 coding exons of MYB plus the alternatively spliced exon 9A9 as well as the 3 exons of EP300 encompassing the reading frame of the KIX domain were sequenced by standard methods. Peripheral blood DNA from 29 random donors from the Australian Bone Marrow Donor Registry was used to determine allelic frequency in a healthy population.
Sequence analysis revealed 4 separate synonymous single nucleotide polymorphisms in exons 6, 11, 14, and 15 in c-Myb in 7 subjects with ET. Intronic SNPs were also observed close to the intron-exon boundaries of 3 other exons. The presence of these SNPs did not segregate with any disease classification and, where frequent, the incidence of these SNPs was not different from that observed in random donor DNA. One patient was heterozygous for a T-to-G substitution in EP300 exon 10 (GenBank accession no. DQ229147) that was not observed in any other DNA sample but was present in DNA derived from this patient's buccal mucosa. This polymorphism replaces the hydrophobic methionine with the basic arginine at amino acid position 664 and could theoretically interfere with the c-Myb–KIX domain interaction. The woman from whom this sample was collected was diagnosed with PRV at 30 years of age in 1999 at which time she had a normal platelet count. In subsequent years, her platelet count has steadily increased (platelet count, 760 × 109/L in 2005) associated with increasing reticulin fibrosis on serial bone marrow biopsies. The role of this polymorphism in EP300 in the changing phenotype of this woman's JAK2V617F-positive myeloproliferative disease is unclear.
Insight into the pathogenesis of the human myeloproliferative disorders has been greatly advanced by the recent discovery of the JAK2V617F mutation. Inherited differences or acquired mutations in other hematopoietic genes are likely to play a role in phenotypic variation in JAK2V617F-positive MPDs and particularly in those cases that are JAK2V617F negative. Our data and that in the letter from Drs Steensma and Pardanani suggest that a direct role for mutated MYB or EP300 in the pathogenesis of these disorders is uncommon.
Correspondence: Andrew Roberts, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Melbourne, 3050, Australia; e-mail: roberts@wehi.edu.au.
Supported by National Cancer Institute award K12 CA90628 to D.P.S. We thank Terra Lasho and Heather Powell for DNA extraction and cell bank curation, and Scott H. Kaufmann and Ayalew Tefferi for helpful advice.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal