Concurrent activation of the T-cell receptor (TCR) and complement regulator CD46 on human CD4+ T lymphocytes induces Tr1-like regulatory T cells that suppress through IL-10 secretion bystander T-cell proliferation. Here we show that, despite their IL-10 production, CD46-induced T-regulatory T cells (Tregs) do not suppress the activation/maturation of dendritic cells (DCs). DC maturation by complement/CD46-induced Tregs is mediated through simultaneous secretion of GM-CSF and soluble CD40L, factors favoring DC differentiation and reversing inhibitory effects of IL-10. Thus, CD46-induced Tregs produce a distinct cytokine profile that inhibits T-cell responses but leaves DC activation unimpaired. Such “DC-sparing” Tregs could be desirable at host/environment interfaces such as the gastrointestinal tract where their specific cytokine profile provides a mechanism that ensures unresponsiveness to commensal bacteria while maintaining reactivity to invading pathogens.
Introduction
A paramount feature of the immune system is to be able to counter an infectious threat while remaining unresponsive to antigens of healthy tissues and harmless substances. Concerning the latter, a number of mechanisms are in place to ensure such immunologic tolerance. Much interest has been focused on CD4+ T-regulatory T cells (Tregs), which can modulate the activation and function of T helper type 1 (Th1) and Th2 effector cells.1-3 Tregs are currently divided into 2 groups: natural (or thymic) CD4+CD25+ Tregs and adaptive, peripherally induced Tr1 and Th3 cells.2,4 Natural Tregs constitute about 10% of peripheral-blood CD4+ T lymphocytes, express the transcription factor Foxp3 as well as high levels of the IL-2R alpha chain (CD25), and require exogenous IL-2.5,6 These cells originate in the thymus, are specific for self-antigens, and act via a contact-dependent mechanism.2,4 Adaptive Tr1 and Th3 cells are generated in the periphery against both self- and foreign antigens.3 They also require exogenous IL-2 and mediate their suppressive effect by the secretion of immunosuppressive cytokines.7-9 Adaptive Tregs may5 or may not10 express Foxp3 and show variable expression of CD25.3 While Th3 cells secrete TGF-β and seem to be central for the induction of oral tolerance,8,11 Tr1 cells are characterized by high-level synthesis of IL-10 without concurrent IL-4 production7,12 and may play a role in the maintenance of tolerance against enteric bacteria.7,13-15 The interrelationships among Treg subpopulations are poorly understood and recent work suggests the existence of additional adaptive human Treg-cell types.16
We recently discovered that CD46, a widely expressed complement inhibitor, functions as a costimulator in the activation of human peripheral CD4+ T cells. Cross-linking of CD46 with either its natural ligands, such as C3b dimers17 or the CD46-binding streptococcal M protein,18 or with mAbs17 in the presence of T-cell receptor (TCR) stimulation leads to the development of highly proliferative cells with a regulatory phenotype resembling Tr1 cells.17,18 CD3/CD46-induced Tregs synthesize large amounts of IL-10 and low or undetectable quantities of IL-2, IL-4, and IL-5. They are able to suppress the activation of bystander T cells in an IL-10–dependent, contact-independent manner.17 Their induction requires exogenous IL-217 but no pre-existing basal expression of Foxp3.19 Of interest, in vitro–generated CD46-induced Tr1-like cells also demonstrate strong granzyme B and perforin expression and display contact-dependent cytotoxicity toward autologous immunocompetent T cells, including activated CD4+ and CD8+ T cells.19,20 Thus, Tr1-like cells generated through CD46 activation possess 2 distinct mechanisms for T-cell suppression: secretion of IL-10 and granzyme B–mediated contact-dependent inhibition.
The effect of Tregs on T-lymphocyte activation and function has received much attention, while modulation of other human-immunocompetent T-cell populations has been less studied. However, an area of growing interest is the communication between Tregs and dendritic cells (DCs), where a “feedback loop” relationship has been proposed: namely, immature DCs or IL-10–treated DCs induce the development of anergic or regulatory T cells,21,22 which then inhibit DC maturation.21,23,24 IL-10 inhibits the terminal differentiation of immature DCs into mature DCs by preventing the up-regulation of MHC class II and the costimulators CD80 and CD86.22,25 Such IL-10–treated DCs fail to synthesize IL-12 upon LPS stimulation and show only limited capacity in the MLR.25 This model of perpetual induction of T-cell suppression has been called “infectious tolerance.”26
Here, we analyze the effect of supernatants derived from human CD3/CD46-activated Tr1-like cells on DC maturation and function. These supernatants suppressed the activation of bystander T cells through IL-10 but, surprisingly, did not block the differentiation of DCs. Analysis of the Tr1-cell supernatants revealed that, in addition to IL-10, CD46-induced Tr1-like cells secrete high amounts of GM-CSF and soluble CD40 ligand (CD40L, CD154), mediators of DC differentiation/maturation.27,28 Neutralization of CD40L in the Treg supernatants or in supernatants derived from a CD40L-deficient patient abrogated DC maturation. Thus, through their unique cytokine profile, complement-induced Tr1-like cells downmodulate T-cell responses but simultaneously permit DC maturation. These features set CD46-induced Tregs apart from other adaptive Treg populations that suppress DC differentiation.
Patients, materials, and methods
Donors and statistical analyses
Donors. Blood from 8 healthy donors and 1 CD40L-deficient patient was collected/used according to the Washington University Medical Center Human Studies Committee guidelines. Informed consent was obtained from all donors. The CD40L-deficient patient is a 19-year-old African-American man who was diagnosed with HIGML (X-linked immunodeficiency with hyper IgM) at age 3 years. We confirmed the lack of CD40L surface expression or secretion by blood cells using fluorescence-activated cell sorter (FACS) analysis and enzyme-linked immunosorbent assay (ELISA; BioSource International, Camarillo, CA), respectively. Experiments involving healthy donors were performed at least 3 times using a different donor each time and all activation conditions were performed in triplicate. Interdonor variability for cytokine secretion ranged from 5% to 30%.
Statistical analyses. The proliferation count data were examined by analysis of variance (ANOVA) after log transformation, which is appropriate for Poisson distributed count data. The data from the different experiments were pooled to obtain an estimate of within-cell variance, and differences between groups were obtained using planned comparisons. The planned contrasts all involved single comparisons with one degree of freedom. However, the comparisons between conditions needed to be done in a manner in which each of the experiments and dilution levels was treated separately. The solution lay in performing the comparisons separately for each experiment and dilution level, using only the numerator DF appropriate to the comparison in question. To interpret the results, data from different dilutions and experiments were standardized separately, using the value for CD3 (Figures 1C, 3B) or CD3/CD28 (Figure 4A) as the standard; standardization was done as a simple ratio.
Media and antibodies
Media and supplements were obtained from the tissue culture facility at Washington University School of Medicine. T cells were maintained in RPMI-1640 medium (Gibco, Carlsbad, CA) with 15% FCS and 200 mM l-glutamine in the presence of 40 U/mL recombinant human IL-2 (BioSource International), and monocytes/DCs were cultured in RPMI-1640 supplemented with FCS, l-glutamine, sodium pyruvate, nonessential amino acids (Gibco), 50 ng/mL GM-CSF (Leukine Berlex, Wayne, NJ), and 1000 U/mL IL-4. Recombinant IL-10 was purchased from BD PharMingen (San Diego, CA). The mAb to CD46 used in this study, TRA-2-10, recognizes an epitope within the first complement control repeat.29 The mAbs to human CD3 (HIT3a) and CD28 (CD28.2) used for T-cell activation and the function neutralizing mAbs to human IL-10 (JES3-9D7), GM-CSF (BVD2-21C11), and TNF-α (MAb1) were purchased from BD PharMingen. The function-neutralizing mAb to human CD40L/CD15424-31 was from eBioscience (San Diego, CA). The mouse nonspecific isotype-matched control mAb (MOPC 31c, IgG1) was obtained from Sigma-Aldrich (St Louis, MO). Human CD4 and CD14 MicroBeads (Miltenyi Biotec, Auburn, CA) were used to isolate peripheral CD4+ T lymphocytes or monocytes, respectively. Monoclonal Abs to human CD3 (HIT3a), CD4 (RPA-T4), CD45RA (HI100), and CD45RO (UCHL1), labeled with FITC, PE, allophycocyanin, or PerCP, were used for cell sorting and obtained from BD Bioscience. Primary-labeled mAbs to human CD80, CD86, and HLA-DR were purchased from BD PharMingen. Abs to human CD1a, CD14, CD16, and CD83 were obtained from Immunotech (Miami, FL).
Purification and stimulation of CD4+ lymphocytes
CD4+ T lymphocytes and subpopulations thereof were purified from whole blood as previously described.17 In vitro stimulation of isolated CD4+ T cells was performed in 96-well plates coated with mAbs to CD3 (10 μg/mL) and CD28 (5 μg/mL), CD46 (5 μg/mL), or a matched IgG1 isotype control Ab (5 μg/mL). The wells were washed, and purified CD4+ lymphocytes (1.5-2.0 × 105 cells/well) were added in 100 μL culture medium supplemented with 20 to 50 U/mL recombinant human IL-2. The plates were centrifuged at 250g for 1 minute and incubated at 37°C in 5% CO2, and supernatants were harvested at desired time points. T-cell proliferation during the bystander T-cell suppression experiments was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay from Promega (Madison, WI). This colorimetric assay determines the number of living cells using an MTS tetrazolium compound (Owen reagent). Initial experiments obtained equivalent results using this assay compared with [3H] thymidine incorporation.
Both naive CD4+ T cells (CD45RA+/RO-) and T cells with a CD45RA+/RO+ phenotype respond to primary CD46 activation with IL-10 production.17 The latter subpopulation shows an earlier and stronger response (referred to as “high responders”).17 In order to minimize the effect of cytokines secreted by restimulated memory effector T cells and endogenous pre-existing Tregs contained in the CD45RA-/RO+ fraction, we performed all experiments with supernatants derived from sorted naive and high-responder CD4+ T cells combined, excluding the memory T-cell population.
Purification and stimulation of monocytes/iDCs
Monocytes were isolated from peripheral-blood mononuclear cells (PBMCs) via positive selection using Human CD14 MicroBeads (Miltenyi Biotec). To generate immature DCs, monocytes were cultured in 24-well culture plates (2.5 × 105 cells/well) for 3 days in RPMI-1640 supplemented with 50 ng/mL GM-CSF and 1000 U/mL IL-4. Maturation of DCs was then induced by the addition of 100 ng/mL LPS (Salmonella abortus equi; Sigma, St Louis, MO) for 24 hours.
Mixed lymphocyte reaction (MLR)
Immature DCs were incubated in 24-well culture plates for 48 hours with supernatants derived from CD4+ T cells that had been activated for 3 days with mAbs to CD3, CD3/CD28, CD3/CD46, or CD3/isotype control in the presence of IL-2. Additional controls consisted of maintaining immature DCs in media containing IL-4 and GM-CSF or with 10 ng/mL recombinant human IL-10. To induce DC maturation, LPS was added to the cultures during the final 24 hours. The mature DCs were washed, seeded into 96-well culture plates in serial 2-fold dilutions beginning with 1 × 105 cells/well, and irradiated at 20 Gy. Freshly purified allogeneic PBMCs were then added at 1 × 105 cells/well and the cocultures incubated for 3 to 4 days. Proliferation of allogeneic T cells was measured via 18-hour [3H] thymidine incorporation by scintillation spectroscopy.
Cytokine analyses
CD4+ cells (1.5-2.0 × 105 cells/well) were incubated for up to 5 days in 96-well plates coated with mAbs. The secretion of IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IFN-γ, TNF-α, and GM-CSF was assessed in the supernatants with the ProteoPlex 16-Well Human Cytokine Array (EMD Biosciences, San Diego, CA), according to the manufacturer's protocol. In addition, IL-7, IL-10, IL-15, TNF-α, GM-CSF, and CD40L secretion was also measured using the appropriate EASIA kits from BioSource International. For intracellular cytokine staining, T cells (1.5-2.0 × 105 cells/well) were activated with immobilized mAbs for 18 hours. Monensin (eBioscience) was added for the last 8 hours of culture. After appropriate surface marker staining, cells were fixed and permeabilized with eBioscience Permeabilization Buffer and IC Fixation Buffer and then stained for intracellular cytokines. The cytokine secretion profile of the proliferating allogeneic PBMCs in the MLR was analyzed using the BD CBA Th1/Th2 Kit I from Becton Dickinson (San Diego, CA), according to the manufacturer's protocol. Supernatant samples analyzed in this assay were collected from the MLR with the 6250 DC count.
Results
Tr1-like cell supernatants support DC maturation
Human naive CD3/CD46-activated CD4+ T cells develop a cytokine-secreting phenotype distinct from that of T cells activated classically via CD3/CD28 stimulation.17 Most notably, CD46-induced cells produce large amounts of IL-10 and through this cytokine suppress the antigen-specific proliferation of bystander CD4+ T cells. The suppressive effect of these cells on other T cells is reversed by neutralization of IL-1017 (Supplemental Figure S1, available at the Blood website; click on the Supplemental Figure link at the top of the online article). IL-10 is immunosuppressive for proliferating T cells and inhibits the maturation of DCs.22,25 To determine the effects of CD46-induced Tr1-cell cytokines on DCs, human immature DCs were incubated with CD46-induced T-cell supernatants and the DC maturation state and function assessed (Figure 1A). The results were compared with the effects of supernatants derived from T cells activated through CD3 and CD28.
Monocyte-derived DCs differentiated in the presence of GM-CSF/IL-4 display an immature phenotype (MHC class II low, CD86 low) and are fully matured (MHC class II high, CD86 high) by the addition of LPS (Figure 1B, panel 2). If recombinant IL-10 (500 pg/mL) was added during the last 48 hours of culture, DCs showed decreased viability and, as expected, failed to mature upon the addition of LPS (Figure 1B, panel 3). Surprisingly, DCs cultured in the presence of supernatants derived from CD3/CD46-activated T cells demonstrated a mature phenotype (Figure 1B, panel 6). Up-regulation of MHCII/CD86 occurred despite a several fold higher amount of IL-10 in these samples (Table 1) compared with a control condition containing 500 pg/mL IL-10 (Figure 1B, panel 3). Moreover, DCs matured under these conditions produced IL-12 in amounts comparable with those generated in the control media and were also morphologically indistinguishable from those differentiated in the presence of supernatants from CD4+ T cells stimulated with CD3/CD28 or CD3 alone (data not shown). Similar to DCs incubated with GM-CSF/IL-4 control media, Treg-exposed DCs also expressed the additional maturation markers CD1a, CD16, and CD83 and downmodulated monocyte-specific CD14 expression. Also monocytes and DCs express CD46. As a control, we incubated monocyte-derived immature DCs with IL-2 and anti-CD46 antibodies, which did not alter the DC marker expression profile (data not shown). Thus, the observed effects cannot be attributed to direct cross-linking of CD46 on DCs or engagement of Fc receptors.
Cytokine profile of human CD4+ T lymphocytes following CD3/CD28 or CD3/CD46 stimulation
. | Activation condition . | . | . | ||
|---|---|---|---|---|---|
| Cytokines, pg/mL . | CD3 . | CD3/CD28 . | CD3/CD46 . | ||
| IL-1α* | — | 30 ± 1.5 | 20 ± 1.0 | ||
| IL-1β* | — | — | — | ||
| IL-2* | — | 500 ± 100 | — | ||
| IL-4* | — | 50 ± 2.0 | — | ||
| IL-6* | — | — | — | ||
| IL-7* | — | — | — | ||
| IL-8* | 30 ± 3.0 | 100 ± 15 | 350 ± 65 | ||
| IL-10* | — | 250 ± 50 | 1800 ± 300‡ | ||
| IL-12† | — | — | — | ||
| IL-15† | 40 ± 2 | 70 ± 2 | 20 ± 2.0 | ||
| TNF-α* | 50 ± 8.0 | 200 ± 30 | 600 ± 100‡ | ||
| IFN-γ* | 80 ± 14 | 200 ± 40 | 900 ± 150‡ | ||
| GM-CSF* | 100 ± 10 | 300 ± 40 | 2200 ± 400‡ | ||
| Soluble CD40L (CD154)† | — | 80 ± 5 | 1300 ± 190‡ | ||
. | Activation condition . | . | . | ||
|---|---|---|---|---|---|
| Cytokines, pg/mL . | CD3 . | CD3/CD28 . | CD3/CD46 . | ||
| IL-1α* | — | 30 ± 1.5 | 20 ± 1.0 | ||
| IL-1β* | — | — | — | ||
| IL-2* | — | 500 ± 100 | — | ||
| IL-4* | — | 50 ± 2.0 | — | ||
| IL-6* | — | — | — | ||
| IL-7* | — | — | — | ||
| IL-8* | 30 ± 3.0 | 100 ± 15 | 350 ± 65 | ||
| IL-10* | — | 250 ± 50 | 1800 ± 300‡ | ||
| IL-12† | — | — | — | ||
| IL-15† | 40 ± 2 | 70 ± 2 | 20 ± 2.0 | ||
| TNF-α* | 50 ± 8.0 | 200 ± 30 | 600 ± 100‡ | ||
| IFN-γ* | 80 ± 14 | 200 ± 40 | 900 ± 150‡ | ||
| GM-CSF* | 100 ± 10 | 300 ± 40 | 2200 ± 400‡ | ||
| Soluble CD40L (CD154)† | — | 80 ± 5 | 1300 ± 190‡ | ||
Purified peripheral T cells (CD4+/CD45RA+) were activated with the indicated antibodies, supernatants were collected at day 3, and secreted cytokines were measured using the *ProteoPlexTM 16-Well Human Cytokine Array (Novagen, Madison, WI) or †ELISA. The expression of IL-10, TNF-α, IFN-γ, and GM-CSF was also confirmed by ELISA (not shown). Activation conditions were performed in triplicate. Data are the mean ± SD of 5 independent experiments using 5 different donors.
— indicates not detectable (cytokine content < 5 pg/mL)
The observed level of statistical difference in cytokine production between CD3/CD28- and CD3/CD46-activated cells was P < .001 by the paired Student t test in all cases
CD46-induced Tr1-like cell supernatants do not suppress dendritic-cell (DC) maturation despite high IL-10 content. (A) General experimental approach. Purified human blood monocytes were cultured in GM-CSF/IL-4–containing media for 72 hours. The generated DC precursors were incubated for 24 hours with supernatants derived from either CD3-, CD3/CD28-, or CD3/CD46-activated T cells (Tr1) or maintained in GM-CSF/IL-4–containing control media. Maturation of these DC populations was induced by LPS addition for 24 hours. The maturation stage of the DCs was determined by their maturation marker expression profile and their potential to induce allogeneic T-cell proliferation in an MLR. (B) DCs exposed to supernatants from CD3/CD46-activated Tr1 cells up-regulate maturation markers. iDCs were generated and the media replaced with supernatants derived from CD3/CD46-induced Tr1 cells, CD3- and CD3/CD28-activated CD4+ T cells, or fresh media with 500 pg/mL recombinant human IL-10 (rec. IL-10). DC maturation was induced by LPS addition and surface expression of MHCII, and CD86 was analyzed by FACS after 24 hours. (C-D) DCs matured in control media (C) or in supernatants from CD3/CD46-activated Tr1 cells (D) demonstrate strong MLR potential. DCs were generated and treated as described in panel B, and their potential to induce allogeneic T-cell proliferation was measured in an MLR: DCs were seeded in serial 2-fold dilutions beginning with 50 × 104 cells/well and irradiated. Purified allogeneic PBMCs were then added at 50 × 104 cells/well, the cocultures were incubated for 4 days, and proliferation of allogeneic T cells was measured via [3H] thymidine incorporation. Shown is 1 representative of 8 independently performed experiments (with each activation condition in triplicate). (E) Summary and statistical evaluation of all proliferation experiments is shown as relative data ± standard error. n indicates the number of separate proliferation conditions (ie, 4 dilutions/experiment in duplicates or triplicates) considered for this evaluation. Mo indicates monocyte; Tr1 supern., supernatant derived from CD3/CD46-induced Tr1-like cells; and MLR, mixed lymphocyte reaction. F statistics and P values are shown for the comparison.
CD46-induced Tr1-like cell supernatants do not suppress dendritic-cell (DC) maturation despite high IL-10 content. (A) General experimental approach. Purified human blood monocytes were cultured in GM-CSF/IL-4–containing media for 72 hours. The generated DC precursors were incubated for 24 hours with supernatants derived from either CD3-, CD3/CD28-, or CD3/CD46-activated T cells (Tr1) or maintained in GM-CSF/IL-4–containing control media. Maturation of these DC populations was induced by LPS addition for 24 hours. The maturation stage of the DCs was determined by their maturation marker expression profile and their potential to induce allogeneic T-cell proliferation in an MLR. (B) DCs exposed to supernatants from CD3/CD46-activated Tr1 cells up-regulate maturation markers. iDCs were generated and the media replaced with supernatants derived from CD3/CD46-induced Tr1 cells, CD3- and CD3/CD28-activated CD4+ T cells, or fresh media with 500 pg/mL recombinant human IL-10 (rec. IL-10). DC maturation was induced by LPS addition and surface expression of MHCII, and CD86 was analyzed by FACS after 24 hours. (C-D) DCs matured in control media (C) or in supernatants from CD3/CD46-activated Tr1 cells (D) demonstrate strong MLR potential. DCs were generated and treated as described in panel B, and their potential to induce allogeneic T-cell proliferation was measured in an MLR: DCs were seeded in serial 2-fold dilutions beginning with 50 × 104 cells/well and irradiated. Purified allogeneic PBMCs were then added at 50 × 104 cells/well, the cocultures were incubated for 4 days, and proliferation of allogeneic T cells was measured via [3H] thymidine incorporation. Shown is 1 representative of 8 independently performed experiments (with each activation condition in triplicate). (E) Summary and statistical evaluation of all proliferation experiments is shown as relative data ± standard error. n indicates the number of separate proliferation conditions (ie, 4 dilutions/experiment in duplicates or triplicates) considered for this evaluation. Mo indicates monocyte; Tr1 supern., supernatant derived from CD3/CD46-induced Tr1-like cells; and MLR, mixed lymphocyte reaction. F statistics and P values are shown for the comparison.
To assess the immune stimulatory function of DCs matured in the presence of supernatants from CD46-induced Tregs, we performed allogeneic MLRs. DCs were matured using the standard protocol of culturing with GM-CSF/IL-4 followed by terminal differentiation with LPS. These cells induced potent proliferation of allogeneic T cells, which was abrogated if recombinant IL-10 was added during DC differentiation (Figure 1C). Allogeneic T-cell proliferation was enhanced by DCs that had been previously cultured in the presence of supernatant from CD3 or CD3/CD28-stimulated T cells, and similarly if DCs were cultured in CD3/CD46-activated Tr1-like cell supernatants (Figure 1D). Comparable results were obtained with all donor pairings in 8 independent experiments. Figure 1E summarizes and statistically evaluates the relative proliferation for all experiments performed. These results suggest that the immunosuppressive effect of IL-10 on DC maturation was offset by additional factors in the supernatant of CD3/CD46-activated T cells.
Tr1-like cells secrete soluble CD40L and GM-CSF
As part of an ongoing effort in our laboratory, we performed a differential gene expression analysis using Affymetrix microchip arrays (Affymetrix, Santa Clara, CA) with RNA from CD3/CD28- and CD3/CD46-stimulated human CD4+ T cells. We found that CD3/CD46-activated cells contain about 3 times higher RNA levels coding for CD40 ligand (CD40L; CD154) compared with CD3/CD28-activated cells (data not shown). We confirmed increased CD40L protein expression by CD46-activated T cells via FACS analysis (Figure 2A). CD40L is a cell-surface molecule on activated T cells30 ; however, biologically active CD40L cleavage products (sCD40L) can also be secreted by these cells.31 A subsequent analysis of supernatants from activated T cells using a cytokine protein array and ELISA revealed that, besides IL-8, IL-10, TNF-α, IFN-γ, GM-CSF, and soluble CD40L (sCD40L) levels were elevated in supernatants from CD3/CD46-activated T cells (Table 1). GM-CSF is a growth factor for mature macrophages and granulocytes and stimulates DC activation,27,32 even in the presence of IL-10.33 CD40L is a potent inducer of dendritic-cell maturation28,34 and is required for the IgM/IgG class switching by B-lymphocyte blasts.35
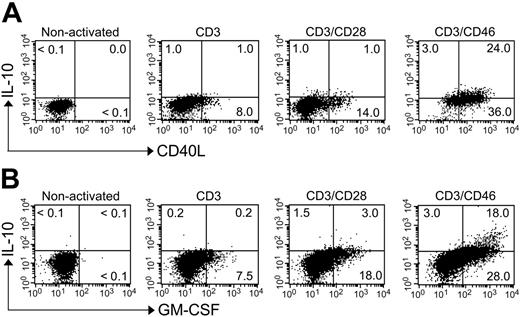
About 40% of cultured human peripheral CD4+ T cells synthesize IL-10 upon CD3/CD46 stimulation.17,20 To determine whether these Tregs also secrete sCD40L and/or GM-CSF, we performed intracellular staining for IL-10/CD40L (Figure 2A) and IL-10/GM-CSF (Figure 2B). Almost all CD46-induced IL-10–secreting cells stained positively for CD40L or GM-CSF. However, the expression/secretion of these 3 factors followed different kinetics. Secretion of CD40L and GM-CSF was detected by 8 to 10 hours after activation, with expression levels peaking at 48 to 52 hours (data not shown). In contrast, IL-10 was not detectable in the supernatants until 18 hours and expression peaked at 72 to 78 hours.17 T cells stimulated by CD3 or CD3/CD28 produced much less of these cytokines and sCD40L (Figure 2A-B; Table 1).
Soluble CD40L mediates DC activation by Tr1-like cells
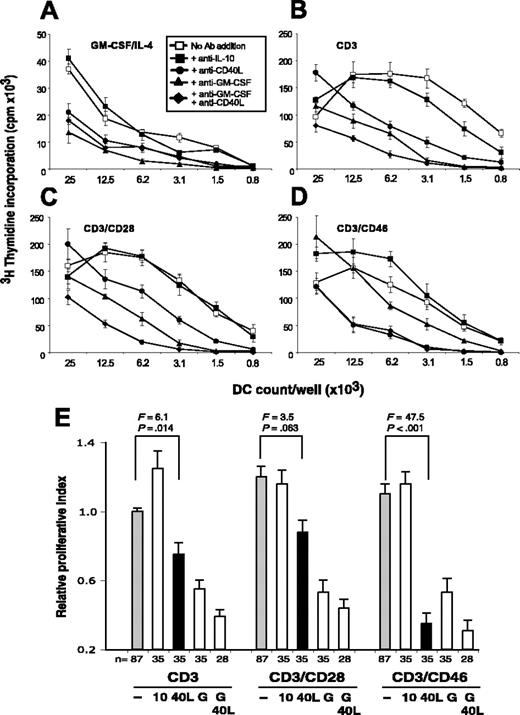
GM-CSF modulates the inhibitory effects of IL-10 on DC maturation under certain conditions,33 and CD40 ligation renders DCs unresponsive to the suppressive effects of natural Tregs.36 Thus, we assessed whether one or both of these factors mediated the observed DC maturation. DCs were incubated in either control media or T-cell–derived supernatants, with or without mAbs to GM-CSF and/or CD40L. To induce terminal DC maturation, LPS was added to the culture and the activation state of DCs assessed in the MLR (Figure 3). GM-CSF neutralization in the control media led to DCs with low viability and little PBMC proliferative capacity (Figure 3A). GM-CSF neutralization also had a strong impact on DCs matured in CD3- and CD3/CD28-activated T-cell supernatants (Figure 3B-C) and a moderate effect on DCs cultured in CD3/CD46-activated T-cell supernatants (Figure 3D). Of interest, while only the neutralization of both GM-CSF and CD40L in the supernatants from CD3- and CD3/CD28-activated T cells led to inhibition of DC maturation, the addition of anti-CD40L alone to supernatants from CD46-induced Tr1-like cells abrogated the DC-mediated T-cell expansion (Figure 3D). A study by MacDonald et al33 indicates that TNF-α reverses the suppressive effect of IL-10 on DC maturation. However, in our experiments the neutralization of TNF-α had no significant effect on DC maturation and the subsequent MLR (data not shown). Figure 3E summarizes and statistically evaluates all of the proliferation data obtained in 5 independent experiments. Taken together, these data suggest that soluble CD40L plays a predominant role in inducing maturation and antigen presentation by DCs conditioned in supernatants derived from CD3/CD46 Tregs.
CD46-activated IL-10–secreting CD4+ T cells express high amounts of CD40L and GM-CSF. Purified peripheral-blood T cells (sorted CD4+CD45RA+ cell population) were activated with the indicated immobilized mAbs for 18 hours, and Monensin was added to the last 8 hours of culture. Cells were permeabilized and fixed, and intracellular cytokine staining was then performed for (A) IL-10 and CD40L or (B) IL-10 and GM-CSF. Shown is 1 representative FACS analysis of 3 independently performed experiments (with each activation condition in triplicate). The observed level of significance for the differences in the amount of IL-10/CD40L or IL-10/GM-CSF production between CD3/CD28- and CD3/CD46-activated cells was P < .001 by the paired Student t test in all cases.
CD46-activated IL-10–secreting CD4+ T cells express high amounts of CD40L and GM-CSF. Purified peripheral-blood T cells (sorted CD4+CD45RA+ cell population) were activated with the indicated immobilized mAbs for 18 hours, and Monensin was added to the last 8 hours of culture. Cells were permeabilized and fixed, and intracellular cytokine staining was then performed for (A) IL-10 and CD40L or (B) IL-10 and GM-CSF. Shown is 1 representative FACS analysis of 3 independently performed experiments (with each activation condition in triplicate). The observed level of significance for the differences in the amount of IL-10/CD40L or IL-10/GM-CSF production between CD3/CD28- and CD3/CD46-activated cells was P < .001 by the paired Student t test in all cases.
Soluble CD40L mediates the DC activation in supernatants derived from CD46-induced Tr1-like cells. (A-D) iDCs were incubated with control media containing GM-CSF/IL-4 (A) or supernatants derived from T cells activated with CD3 (B), CD3/CD28 (C), or CD3/CD46 (D). Each condition was performed with or without the addition of the indicated function neutralizing mAbs. A nonspecific isotype mAb was used as a control. This mAb had no effect on any of the tested activation conditions (data not shown). DC maturation was induced with LPS and the capacity of the DCs to induce allogeneic T-cell activation was analyzed in an MLR. Shown is 1 representative of 5 independently performed experiments (with each activation condition in triplicate). (E) Summary and statistical evaluation of all proliferation data obtained in this set of experiments, displayed as described in Figure 1E. F statistics and P values are shown for the comparison between untreated (gray bars) and anti-CD40L ligand mAb–treated conditions (black bars). 10, 40L, and G indicate the addition of function-neutralizing mAbs to IL-10, CD40L, and GM-CSF, respectively.
Soluble CD40L mediates the DC activation in supernatants derived from CD46-induced Tr1-like cells. (A-D) iDCs were incubated with control media containing GM-CSF/IL-4 (A) or supernatants derived from T cells activated with CD3 (B), CD3/CD28 (C), or CD3/CD46 (D). Each condition was performed with or without the addition of the indicated function neutralizing mAbs. A nonspecific isotype mAb was used as a control. This mAb had no effect on any of the tested activation conditions (data not shown). DC maturation was induced with LPS and the capacity of the DCs to induce allogeneic T-cell activation was analyzed in an MLR. Shown is 1 representative of 5 independently performed experiments (with each activation condition in triplicate). (E) Summary and statistical evaluation of all proliferation data obtained in this set of experiments, displayed as described in Figure 1E. F statistics and P values are shown for the comparison between untreated (gray bars) and anti-CD40L ligand mAb–treated conditions (black bars). 10, 40L, and G indicate the addition of function-neutralizing mAbs to IL-10, CD40L, and GM-CSF, respectively.
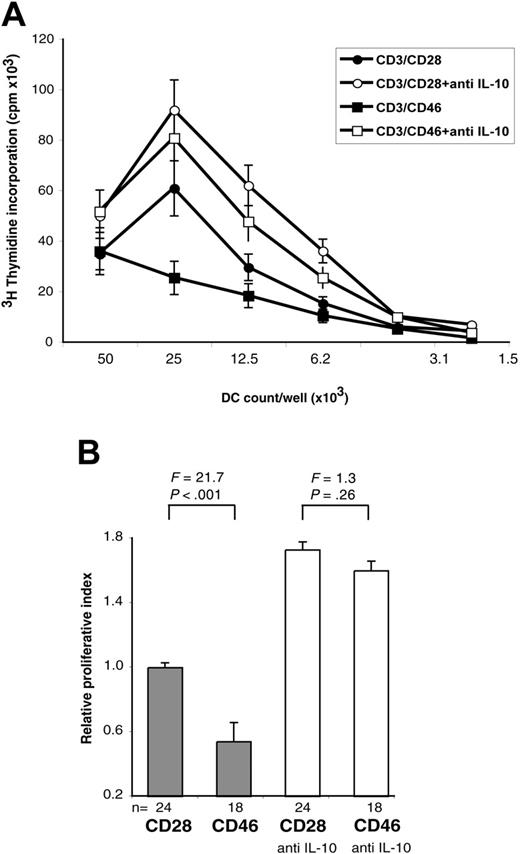
To further investigate the key role of sCD40L, we analyzed supernatants derived from a CD40L-deficient patient. CD40L deficiency is associated with hyper-IgM syndrome (HIGML, X-linked immunodeficiency with hyper IgM) as CD40L is indispensable for the induction of the IgM/IgG class switching by B lymphocytes.37 Lack of cell-surface and soluble CD40L in this patient was ascertained by FACS and ELISA, respectively (data not shown). This patient's CD4+ T cells responded to CD3/CD46 activation with a cytokine secretion profile similar to that observed in healthy donors (ie, strong IL-10 secretion; ∼ 3 ng/mL at day 3) without concurrent IL-4 and IL-2 production (data not shown). However, DCs exposed to these Tr1-like supernatants failed to induce the proliferation of allogeneic PBMCs, while DCs matured in supernatants from CD3/CD28-activated T cells displayed the expected stimulating properties (Figure 4A). Moreover, neutralization of IL-10 in the CD46-induced Tr1-like cell supernatants restored the capacity of DCs to induce PBMC proliferation (Figure 4A). A summary and statistical evaluation of 3 independent proliferation experiments is shown in Figure 4B.
GM-CSF is necessary for the development and maintenance of DCs,25,28 and neutralization of this factor in T-cell culture supernatants decreased their viability and their antigen-presenting capability (Figure 3). CD3/CD46-induced supernatants contain higher amounts of IL-10 than supernatants from CD3- or CD3/CD28-activated T cells. This likely explains why neutralization of sCD40L in the CD3/CD46-activated Treg supernatants renders them suppressive for DC maturation, while CD3/CD28-activated T-cell supernatants still promote DC activation (Figure 3). Similarly, Tr1-like cells from the CD40L-deficient patient did not induce DC maturation, while his CD3/CD28-activated T cells permitted DC activation (Figure 4). Thus, GM-CSF functions as a survival/growth factor for DCs, while sCD40L is the predominant factor that counteracts the immunosuppressive effect of IL-10 on DCs. This interpretation is supported by a recent study demonstrating that a CD40/CD40L interaction releases DCs from IL-10–mediated suppression in vitro,38 as well as suppression by natural CD4+CD25+ Tregs in vivo.36
DCs matured in Tr1-like cell supernatants do not induce the proliferation of IL-10–secreting Treg cells
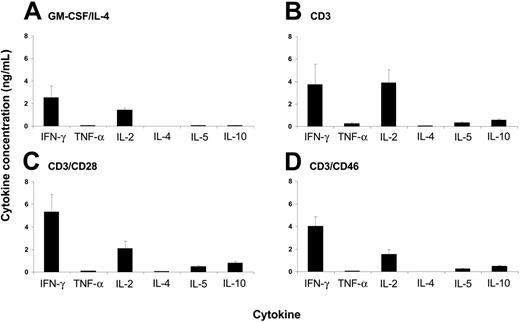
IL-10–treated DCs can induce the development of anergic and regulatory T cells.21,22 Although the DCs matured in the presence of Tr1-like cell supernatants display conventional properties, we analyzed the phenotype of the T cells that expanded through the interaction with such DCs. We performed MLR using DCs matured in supernatants from either CD3-, CD3/CD28-, or CD3/CD46-activated T cells and assessed the cytokine profile of the resulting proliferating MLR cultures for Th1/Th2 cytokines (Figure 5). Irrespective of the supernatant in which the DCs matured, the proliferating T cells produced moderate amounts of IFN-γ and IL-2, but only limited amounts of IL-10. We detected no substantial differences in proliferation rate or the cytokine profile among the analyzed MLR samples (Figure 5B-D). They were similar to that produced by MLRs induced through control DCs (matured in media containing GM-CSF/IL-4) (Figure 5A). Therefore, DCs matured in the presence of cytokines produced by CD3/CD46-activated Tregs did not induce responding T cells to acquire a Treg phenotype but rather stimulated proliferation and the cytokine profile of normal effector T cells.
MLR potential of DCs incubated with CD46-activated T-cell supernatant derived from a CD40L-deficient patient. (A) iDCs were incubated with supernatants derived from CD4+ T cells from a CD40L-deficient patient activated with CD3/CD46 or CD3/CD28, with or without the addition of a function neutralizing mAb to IL-10. The capacity of DCs to induce allogeneic T-cell activation after LPS addition was analyzed in an MLR. Shown is 1 representative of 3 independently performed experiments (with each activation condition in triplicate). (B) Summary and statistical evaluation of all proliferation data obtained in this set of experiments, displayed as described in Figure 1E.
MLR potential of DCs incubated with CD46-activated T-cell supernatant derived from a CD40L-deficient patient. (A) iDCs were incubated with supernatants derived from CD4+ T cells from a CD40L-deficient patient activated with CD3/CD46 or CD3/CD28, with or without the addition of a function neutralizing mAb to IL-10. The capacity of DCs to induce allogeneic T-cell activation after LPS addition was analyzed in an MLR. Shown is 1 representative of 3 independently performed experiments (with each activation condition in triplicate). (B) Summary and statistical evaluation of all proliferation data obtained in this set of experiments, displayed as described in Figure 1E.
Discussion
CD46 is a widely expressed transmembrane glycoprotein that was initially identified as a complement regulator and is also a receptor for several viral and bacterial pathogens.39,40 It binds the opsonins C3b and C4b and serves as a cofactor in their proteolytic cleavage by factor I. This process, called cofactor activity, is fundamental in protecting host tissues from complement attack39 and is exemplified by the recent finding that heterozygous deficiency of CD46 predisposes to hemolytic uremic syndrome (HUS).41 In addition, CD46 participates in the sperm/egg interaction during fertilization39,42 and functions as a costimulator during T-cell activation. CD3/CD46 cross-linking on human CD4+ T lymphocytes induces intracellular signaling events39 that lead to T-cell proliferation43 and IL-10 production.17 These cells in turn suppress bystander T-cell proliferation through IL-10, a hallmark of T-regulatory type 1 (Tr1) cells.
In this study, we extended our analysis of the cytokine expression profile of CD46-induced Tregs and explored the effects of those cytokines on the activation and maturation of DCs. CD28 is considered the most important T-cell costimulator. T cells activated in vitro via CD3/CD28 proliferate, produce a characteristic spectrum of Th1 and/or Th2 cytokines including IL-2, IFN-γ, and IL-4 (Table 1), and normally do not suppress T-cell or DC activation (Supplemental Figure S1; Figure 3C). CD3/CD46-activated T cells produce little or no IL-2 and IL-4, moderate quantities of IFN-γ, and high amounts of IL-1017 (Table 1). Here we demonstrate that these cells also secrete 7- to 16-fold higher quantities of GM-CSF and sCD40L compared with CD3/CD28-activated T cells. Natural ligands for CD46, the opsonins C3b and C4b, either surface immobilized or as dimers in the fluid phase, induce Tregs with the described cytokine profile and T-cell–suppressive properties.17 Thus, we postulate that the activation of human CD4+ T lymphocytes in the presence of these complement components drives a T-cell phenotype with immunomodulatory properties and a cytokine profile distinct from that of classically CD3/CD28-activated T cells. Suppression of T-cell responses could be a reason why many pathogens including measles virus, herpes virus 6, some group B and D adenoviruses, Streptococcus pyogenes, and the pathogenic Neisseria have evolved to interact with CD46.39,40 This hypothesis is supported by the observation that purified streptococcal M proteins, pathogenic ligands for CD46, as well as whole streptococci, induce these Tr1-like suppressor cells and thereby provide a more conducive environment for an infection.18
DCs matured in the presence of Tr1-like cell supernatants induce the proliferation of conventional effector T cells. Peripheral-blood monocytes were cultured for 3 days in media containing GM-CSF and IL-4. These immature DCs were then incubated for 24 hours in control media containing GM-CSF/IL-4 (A), and supernatants from T cells activated with CD3 (B), CD3/CD28 (C), or CD3/CD46 (D), and maturation was induced by LPS addition for 24 hours. The matured DCs were washed and used in an MLR with allogeneic PBMCs. The cytokine profile of the proliferating PBMCs was analyzed after 5 days using the Th1/Th2 cytokine bead array (BD Biosciences). Data shown represent the mean cytokine production ± SD of 3 independent experiments performed in triplicate. The observed level of significance for the differences in the amount of cytokines produced by DCs treated with either control media or different supernatants was P > .15 by the paired Student t test in all cases.
DCs matured in the presence of Tr1-like cell supernatants induce the proliferation of conventional effector T cells. Peripheral-blood monocytes were cultured for 3 days in media containing GM-CSF and IL-4. These immature DCs were then incubated for 24 hours in control media containing GM-CSF/IL-4 (A), and supernatants from T cells activated with CD3 (B), CD3/CD28 (C), or CD3/CD46 (D), and maturation was induced by LPS addition for 24 hours. The matured DCs were washed and used in an MLR with allogeneic PBMCs. The cytokine profile of the proliferating PBMCs was analyzed after 5 days using the Th1/Th2 cytokine bead array (BD Biosciences). Data shown represent the mean cytokine production ± SD of 3 independent experiments performed in triplicate. The observed level of significance for the differences in the amount of cytokines produced by DCs treated with either control media or different supernatants was P > .15 by the paired Student t test in all cases.
Immature DCs are susceptible to IL-10–mediated suppression.22,25 Therefore, we expected that Tr1-like cell supernatants would inhibit the maturation of DCs. Surprisingly, however, immature DCs treated with these supernatants showed normal up-regulation of maturation markers. In addition, DCs matured in the presence of complement-induced Treg cytokines induced strong allogeneic T-cell proliferation in the MLR, and the proliferating T cells showed normal effector-cell properties. The effect of complement-induced Tregs on DCs is reversed by the neutralization of sCD40L. Thus, CD3/CD28- and CD3/CD46-activated T cells induce antigen-presenting cells (APCs) with similar properties but for different reasons: CD3/CD28-activated T cells produce a cytokine profile with high levels of IL-2 and low levels of suppressive cytokines and directly support both T-cell and DC activation. In contrast, CD3/CD46-activated T cells consume IL-2 and suppress T-cell activation via IL-10 but counteract the normally suppressive effect of this cytokine on DC maturation through the simultaneous secretion of GM-CSF and soluble CD40L.
Coengagement of the TCR and CD46 is one of several protocols to generate Tr1-like cells in vitro. The original Tr1 cells were produced by Groux et al through repeated CD3/CD28 stimulation of purified CD4+ T cells in the presence of IL-107 or later through the treatment of CD4+ T cells with a combination of vitamin D3 and dexamethasone.11 IL-10–secreting CD4+ Tregs have also been produced through the incubation with IL-10 and IFN-α and the interaction with immature or tolerogenic dendritic cells or natural CD4+CD25+ Tregs.3-5 Some Tr1-cell subsets generated by these means inhibit DC maturation and promote infectious tolerance.4,23,26 Thus, the Treg-suppressed immature DCs can in turn generate new anergic or suppressive T cells, initiating a feedback loop leading to the perpetual induction of Tregs.26 In contrast, CD3/CD46-induced IL-10–secreting Tregs (which may still be generated by tolerogenic DCs) suppress effector T-cell responses but allow DC maturation and therefore do not support infectious tolerance. We currently do not understand these functional differences. Recent studies though indicate the existence of several distinct human IL-10–producing Treg lines,16 with their induction dependent on the nature of the antigen, the APC, and the microenvironment during T-cell activation. Because complement-induced human Tregs show overlapping features with Tr1 cells but also acquire novel characteristics, they potentially represent a unique line of Tregs with a distinct functional repertoire. To determine if the DC-promoting activity is specific for CD46-induced adaptive Tregs, we extended our analysis to natural CD4+CD25+ Tregs, which usually inhibit DC maturation.23,26 Natural Tregs were stimulated with CD3/IL-2 or CD3/CD46 and analyzed for their cytokine production. While these cells secreted moderate amounts of GM-CSF, little or no IL-10 or sCD40L was found (data not shown). Whether lack of sCD40L production or a different mechanism accounts for the DC-suppressive properties of natural Tregs remains to be defined.
How Tr1 cells are induced in vivo and in what situation and/or location the required conditions could be met are some of the critical questions. In the following discussion, we speculate on the induction conditions and potential physiologic roles of complement-induced DC-sparing Tregs. Generation of CD46-mediated Tregs is dependent on IL-2 and complement activation fragments, reactants generated during the induction of an immune/effector T-cell response against invading pathogens. Thus, complement-induced Tregs, generated during the expansion of effector T cells, could control the inflammatory response and prevent or limit host tissue damage.9,44
Besides such a more general role in the control and contraction of a successful T-cell response, complement-induced Tregs may participate in the maintenance of mucosal tolerance and immunity. Tr1 cells or IL-10–producing Tregs are relatively abundant in the intestinal tract of humans and mice where they are proposed to maintain tolerance to the enteric gut flora.14,45,46 Altered regulation of the intestinal T-cell populations results in chronic intestinal inflammation.47,48 In addition, IL-2– and IL-10–deficient mice succumb to colitis,49,50 and inflammatory bowel disease (IBD) patients contain proportionally less IL-10–secreting Tregs in the lamina propria compared with healthy individuals.51 The intestinal immune system must be able to mount immune responses against invading pathogens while maintaining tolerance toward commensal bacteria and digested innocuous antigens. Enteric intestinal bacteria are a constant source of foreign antigen, nidus for complement activation, and promoter of inflammatory signals. T cells that encounter their cognate antigen at this location may be induced toward a CD46-induced Treg phenotype. Such a Treg cell pool, sensitized to antigens derived from the normal gut flora, could control effector T-cell–mediated inflammation through local IL-10 secretion.14,47 Of interest, a high number of CD3/CD46-activated Treg cells express the α4β7 integrin and stain positive for OX-40 (data not shown), features of intestinal lamina propria and intraepithelial lymphocytes.52
CD46-induced adaptive Tregs express high amounts of granzyme B and can kill autologous activated target T cells.19,20 These cells, if specific for antigens from commensal bacteria, might kill APCs presenting such innocuous antigens and thereby prevent the induction of unnecessary responses. In contrast, immature DCs encountering invading pathogens should not interact with resident Tr1-like cells because these antigens are not recognized by the CD46-induced Treg pool. Thus, these DCs can mature in the presence of complement-induced granzyme B/IL-10/sCD40L-producing Tregs. Activated DCs could then migrate to the draining lymphoid tissues and initiate an immune response. Along this line, CD40L-deficient patients do not develop IBD or colitis, but do suffer from frequent diarrhea.37 CD40L-deficient mice also show no symptoms of IBD but cannot clear adequately the gut-resident parasite Cryptosporidium parvum.53 Thus, in these situations the host may develop a normal Tr1-cell pool but cannot mount optimal or balanced responses against incoming pathogens because of compromised DC activation.
We are currently investigating complement-induced human Tregs in the gut. In an initial experiment, we isolated CD4+ T cells from the small intestinal lamina propria from patients undergoing bariatric surgery. A subset of the lamina propria resident T cells spontaneously secretes IL-10 and sCD40L, without concurrent IL-4 production (data not shown). Thus, T cells with a cytokine profile characteristic of CD46-induced Tregs may reside in the gastrointestinal tract. Further studies will determine if these cells also share other properties of complement-induced Tregs.
In summary, the data presented here further establish a role for the complement system in controlling immune responses.54,55 Specifically, these results add to our understanding of how the balance between unresponsiveness toward self-antigens (and the commensal flora) and the ability to simultaneously mount a protective immune response against invading pathogens may be governed. As the complement system preceded the evolution of T and B lymphocytes, it is not surprising that features of this ancient part of innate immunity have been borrowed to regulate the initiation and deactivation of the adaptive immune response.
Prepublished online as Blood First Edition Paper, October 20, 2005; DOI 10.1182/blood-2005-07-2951.
Supported by the Washington University/Pfizer Biomedical Research Program and the NIH grants RO1 CA 74881 and P30 AR48335-05.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank William Eades and Jacqueline Hughes in the Siteman Cancer Center High Speed Sorter Core Facility for performing the cell sorting and James Verbsky and Skip Virgin for helpful discussions.

![Figure 1. CD46-induced Tr1-like cell supernatants do not suppress dendritic-cell (DC) maturation despite high IL-10 content. (A) General experimental approach. Purified human blood monocytes were cultured in GM-CSF/IL-4–containing media for 72 hours. The generated DC precursors were incubated for 24 hours with supernatants derived from either CD3-, CD3/CD28-, or CD3/CD46-activated T cells (Tr1) or maintained in GM-CSF/IL-4–containing control media. Maturation of these DC populations was induced by LPS addition for 24 hours. The maturation stage of the DCs was determined by their maturation marker expression profile and their potential to induce allogeneic T-cell proliferation in an MLR. (B) DCs exposed to supernatants from CD3/CD46-activated Tr1 cells up-regulate maturation markers. iDCs were generated and the media replaced with supernatants derived from CD3/CD46-induced Tr1 cells, CD3- and CD3/CD28-activated CD4+ T cells, or fresh media with 500 pg/mL recombinant human IL-10 (rec. IL-10). DC maturation was induced by LPS addition and surface expression of MHCII, and CD86 was analyzed by FACS after 24 hours. (C-D) DCs matured in control media (C) or in supernatants from CD3/CD46-activated Tr1 cells (D) demonstrate strong MLR potential. DCs were generated and treated as described in panel B, and their potential to induce allogeneic T-cell proliferation was measured in an MLR: DCs were seeded in serial 2-fold dilutions beginning with 50 × 104 cells/well and irradiated. Purified allogeneic PBMCs were then added at 50 × 104 cells/well, the cocultures were incubated for 4 days, and proliferation of allogeneic T cells was measured via [3H] thymidine incorporation. Shown is 1 representative of 8 independently performed experiments (with each activation condition in triplicate). (E) Summary and statistical evaluation of all proliferation experiments is shown as relative data ± standard error. n indicates the number of separate proliferation conditions (ie, 4 dilutions/experiment in duplicates or triplicates) considered for this evaluation. Mo indicates monocyte; Tr1 supern., supernatant derived from CD3/CD46-induced Tr1-like cells; and MLR, mixed lymphocyte reaction. F statistics and P values are shown for the comparison.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/4/10.1182_blood-2005-07-2951/2/m_zh80040691310001.jpeg?Expires=1769897029&Signature=aFs3hn~t1vFMKQZYOzHoz5I1mKCmHskrvIQ5mouivY8X7HVu0SP1u70b1OyqSQhhk~7lsIpM2vo2qadqZKootWD3-Aurrgd~cC0n0lbtRsi5Gmrl75mTtw09mI8AZRgHRtdTEB9S5UchDCUU9AcIpypb2hoE56i1oalbGEqtdQ1Vk0QhSRrXR5domhzUXU5OL9PhRd6d11aoXYMgFA4e4Hw58odEAU1xfX4mr5ygx-EOyZagT-6hFN1m5bLb8MdBpKIX5jAv8f0MgzG4jouoEGITtUoEOYdB9TaE9r0MZIuwcepyTyTekz1~oryGlwIKYWtKiFa~3D-IiRrCli5jow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal